Table of Contents
Hydrofluoric acid can be prepared by the action of sulphuric acid on fluorspar (calcium fluoride), but glass apparatus cannot be used in the preparation on account of the action of this gas on the glass. Take a small round piece of sheet lead about 3 inches in diameter, and with the pestle press the centre of the lead in a mortar until you have made it cup-shaped. Into this leaden cup put about 5 or 6 grams of powdered fluorspar. Gently warm a glass plate large enough to cover the mouth of the leaden cup, and rub it over either with beeswax or paraffin, so that the plate is covered with a thin even coating of the wax; then trace some design on the glass plate with a sharp-pointed piece of wood or wire, making sure that the lines are drawn quite through the wax, and expose the glass. Now drench the fluorspar with strong sulphuric acid, and place the glass plate over the leaden cup, waxed side downwards, and warm the cup gently; white fumes come off and fill the cup. Allow these fumes to act upon the glass plate for a few minutes, then remove the plate and wash out the contents of the cup. Clean the wax off the glass plate with a cloth, and you will find the design etched into the glass.
CaF2 + H2SO4 = 2HF + CaSO4
SiO2 + 4HF = SiF4 + 2H2O
The white fumes noticed in the cup are those of hydrofluoric acid, which attack the silica in the glass, forming Silicon tetrafluoride.
Silicon fluoride.—Fix up an apparatus similar to that used in the preparation of carbon dioxide, allowing the flask to rest on a retort-stand. Into the flask put a mixture of about 10 grams of powdered fluorspar and 15 grams of fine sand; pour a little strong sulphuric acid down the thistle funnel, and shake the flask to mix the acid well with the mixture, then apply a gentle heat to the flask and collect the gas in a perfectly dry jar by downward displacement. To test when the jar is full bring a lighted taper into the mouth of the jar; if it is extinguished, cap the jar with a greased cover-glass.
2CaF2 + SiO2 + 2H2SO4 = 2CaSO4 + 2H2O + SiF4
Laboratory Experiment I
Take a filter funnel large enough to rest in the mouth of the jar, and attach to it by means of a piece of caoutchouc tubing a small piece of glass tubing drawn out to a fairly fine point, as shown in fig. 33. Pour a little water into the funnel, remove the cover-glass from the jar and allow the funnel to rest in the mouth of the jar. As each drop of water falls from the opening of the narrow tube it seems to become solid, and a stalactite is formed on the end of the tube.
3SiF4 + 4H2O = H4SiO4 + 2H2SiF6
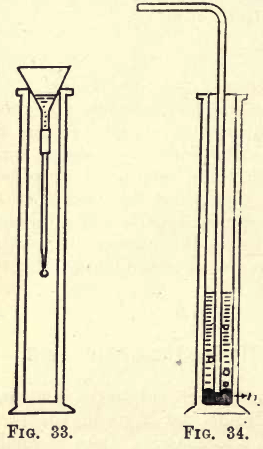
Laboratory Experiment II
Into a gas jar put a little mercury, and allow the delivery tube from the flask to dip just under the mercury (see fig. 34) ; then add a little water, heat the flask gently, and allow the gas to bubble through the mercury into the water; you will notice the silica separating out in the water. After the gas has been passed for a little time filter the water, and test it with a piece of blue litmus paper; it is turned red, showing the presence of an acid, which is hydrofluosilicic acid.
INSTRUCTIONS TO THE STUDENT
The student has now obtained a fair knowledge of simple glass-working and of the preparation and properties of the more common gases. In the following sections he proceeds to examine solids and liquids (generally solids which are put into solution). First he examines an unknown substance by ‘dry tests’ for ‘ bases ’ and ‘ acids ’; the results so obtained are then confirmed by systematic ‘ wet tests.’
The order of work laid down is to be followed with most careful and patient attention to all details; the student has then done his part, but unless this is supplemented by equal care on the part of the demonstrator, the best results cannot be expected. The demonstrator, besides supervising the actual testing, must prepare a carefully graded series of substances leading from simple salts containing one ‘base’ and one ‘ acid ’ to complex mixtures containing four or five ‘bases’ and several ‘ acids ’ (including insolubles). The substances must all be carefully selected with the definite object of teaching the student some important point in every mixture he analyses. Indiscriminate preparation of mixtures leads to waste of time and bad work.
In his first tests the student may be given salts of known composition, and his work is then checked by unknown salts from the demonstrator’s set. For instance, on the next page he may take ZnCl2, SnCl2, Pb, Bi2S3, and so on for practice, and when fairly confident, his proficiency or otherwise is checked on ‘unknown’ salts given by the demonstrator.
The demonstrator’s ‘Record Book’ should show full details of the substances given to each student, the results obtained, the time taken with the analysis, and general remarks where necessary. A somewhat similar record must be kept of the work done in Quantitative Analysis and Assaying.
