Table of Contents
As part of its solid-waste research, the Bureau of Mines is engaged in a broad program to develop low-cost processes for recovering and recycling metals and minerals from a variety of industrial wastes. One benefit is the alleviation of pollution associated with many of these wastes.
The phosphate coating of metals is widely used in industry, especially in the manufacture of steel products. The primary use of phosphate coating is to provide a base to which paint or enamel will strongly adhere, but it is also used for corrosion protection and for lubrication in die-forming operations. The phosphating operation consists of contacting the metal surface by either dipping it in or spraying it with a solution of primary metal phosphates and one or more oxidizing agents in phosphoric acid.
The phosphate coating reaction is based on the insolubility in water and solubility in acids off most metal phosphates. When steel is contacted with the phosphating solution, iron is dissolved, hydrogen is evolved, and the primary metal phosphates are converted to insoluble secondary and tertiary phosphates that deposit on and strongly bond to the metal surface. Because the evolved hydrogen slows the reaction, oxidizing agents are added to convert hydrogen to water. A typical overall reaction is as follows:
![]()
where M is a metal such as iron, zinc, or manganese, and the underlined products are bonded to the metal surface.
The original metal surface is relatively rough, electrically conductive, and susceptible to corrosion; the coated surface is relatively smooth, nonconductive, and corrosion-resistant. Commercial phosphating solutions are usually proprietary and contain other chemicals in addition to those mentioned Furthermore, some metals may be dissolved from the materials undergoing treatment. A concentrated stock solution might be prepared by dissolving zinc in phosphoric acid and adding other ingredients. This solution would be diluted to approximately 3 percent total phosphate for the initial strength of the phosphating solution.
Chemical reaction with iron or steel during the phosphating operation also results in the accumulation of an insoluble sludge in which the main ingredient is ferric phosphate (FePO4). The sludge is collected at intervals by pumping away the supernatant liquid or draining the slurry from the bottom. Phosphate sludges from the treatment of automobile bodies are very fine-grained (100 percent finer than 37 microns and over 50 percent finer than 5 microns). However, they are contaminated by varying amounts of scale and other larger grained debris and by organic matter, including cutting oil, lubricating oil, grease, and various sealants. The composition of the sludges varies from batch to batch. A typical partial chemical analysis of a filtered dried sludge formed in treating automobile bodies is as follows:
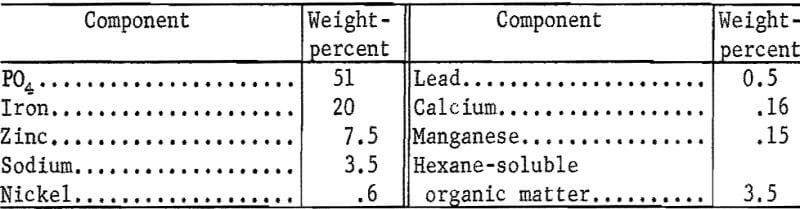
The wastes from phosphating operations are often discharged into streams or landfills. The large quantities of such wastes discharged into streams in the Missouri River Basin have been reported in two Bureau of Mines publications. Based on annual U.S. production of thin sheet steel and assuming that 20 percent of this steel is phosphated, it is estimated that several million gallons of phosphate wastes are dumped each year. This contributes to stream pollution problems, and also represents large losses of zinc and phosphates, together with lesser amounts of other metals.
Phosphorus compounds, such as those in the wastes, are excellent nutrients for algae and other aquatic plants, growth of which causes oxygen depletion in streams and rivers. Even very small quantities of phosphates enhance growth of algae. Also, certain metals in phosphate wastes are toxic to fish and other organisms.
Previous work by the Bureau of Mines on the recovery of phosphates and metals from waste phosphate solutions and sludges has been reported. The purification of phosphoric acid pickle liquors and aluminum bright dip solutions has also been described. The main goal of the research described herein was to provide an alternative method for recovering the values from waste phosphate sludge.
Phosphate sludges contain more kinds and larger quantities of ingredients than do phosphate solutions; therefore, they involve more difficult separation problems. In this work, a major consideration was the large quantity of iron that must be separated from, or treated with, smaller quantities of nickel, zinc, and other metals.
Materials
Phosphate Sludges
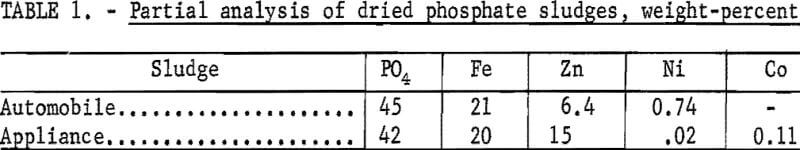
As received, the phosphate wastes contained both solids and liquid. These slurries were filtered, and experimental tests were made on the undried filter cake (sludge). In an industrial process, the solids would probably be recovered by centrifuging, and the liquid would be recycled to the phosphating operation. Partial analyses of filtered and dried phosphate sludges are given in table 1. The sludges were dried at 110° C for 1 hour per 100 grams of undried sludge. The dried sludge still contained the organic matter. The first sludge was produced by phosphating automobile bodies: the second sludge was produced by phosphating steel used in the manufacture of appliances and hardware.
Solvents
The solvent for iron chloride, isopropyl ether (IPE), was a purified grade that was used without diluent or carrier.
The solvent for zinc, a 20-volume-percent solution of di-2-ethylhexyl phosphoric acid (EHPA) in commercial-grade kerosene, was prepared from commercial-grade EHPA. Prior to its first use, the solution was washed with dilute phosphoric acid (pH 2.5) to remove an unidentified material that caused turbidity in the aqueous phase during extraction experiments. It was not necessary to repeat this treatment when the EHPA was recycled.
Chemistry of Extractions
Iron is very efficiently extracted from aqueous hydrochloric acid solutions by IPE, providing that the hydrochloric acid (HCl) concentration is approximately 8 molar. The efficiency of the extraction decreases as the acidity is lowered. The iron is extracted as ferric chloride, and some hydrochloric acid is also extracted. The iron chloride and hydrochloric acid can be easily recovered from the organic phase by stripping with water.
Previous extraction tests indicated that zinc could be selectively extracted from the aqueous, iron-free phosphate solution by EHPA in kerosene. This extraction proceeds by cation exchange between the liquid phases. EHPA exists as a dimer, (RH)2 in kerosene. The generalized equation for the extraction is
![]()
As the equation shows, the progress of the extraction results in increased acidity of the aqueous phase, which was verified experimentally when the pH of the solution dropped from 3.5 to 2.5 during a single extraction. Stripping of the organic phase with dilute (15 volume-percent) sulfuric acid recovers the zinc and regenerates the EHPA.
Experimental Procedures and Results
Automobile Sludge
Dissolution and Extraction of Iron and Zinc
The flowsheet for the sludge dissolution and iron extraction steps is shown in figure 1. In a typical test, 1,000 grams of filtered, undried, phosphate sludge containing 31 percent solids was dissolved in 800 milliliters of hot, concentrated hydrochloric acid. The use of hot acid aided the coalescence and separation of about 35 grams of an oily phase, which must be removed to prevent contamination of the organic solvents. The oily material was separated by skimming and filtration. In practice, skimming and decantation would be used.
The efficient extraction of iron by IPE requires a solution at least 8 normal with respect to hydrochloric acid; therefore, 800 milliliters additional concentrated hydrochloric acid was added to meet this requirement. The volume of the solution was 2.6 liters at this point, and it contained the following (in grams per liter): Phosphate, 56; iron, 27; zinc, 8; and nickel, 0.9. This solution was stirred for 15 minutes with 7.8 liters of IPE in a 5-gallon vessel with a bottom outlet, and the phases were allowed to separate for 15 minutes. Adequate ventilation is required because of the high toxicity of IPE. In addition, protective clothing is required to prevent IPE from contacting the skin. The phases separated cleanly, with no emulsion problems. The iron-free aqueous phase was withdrawn from the bottom of the settler. Only one extraction stage was used.
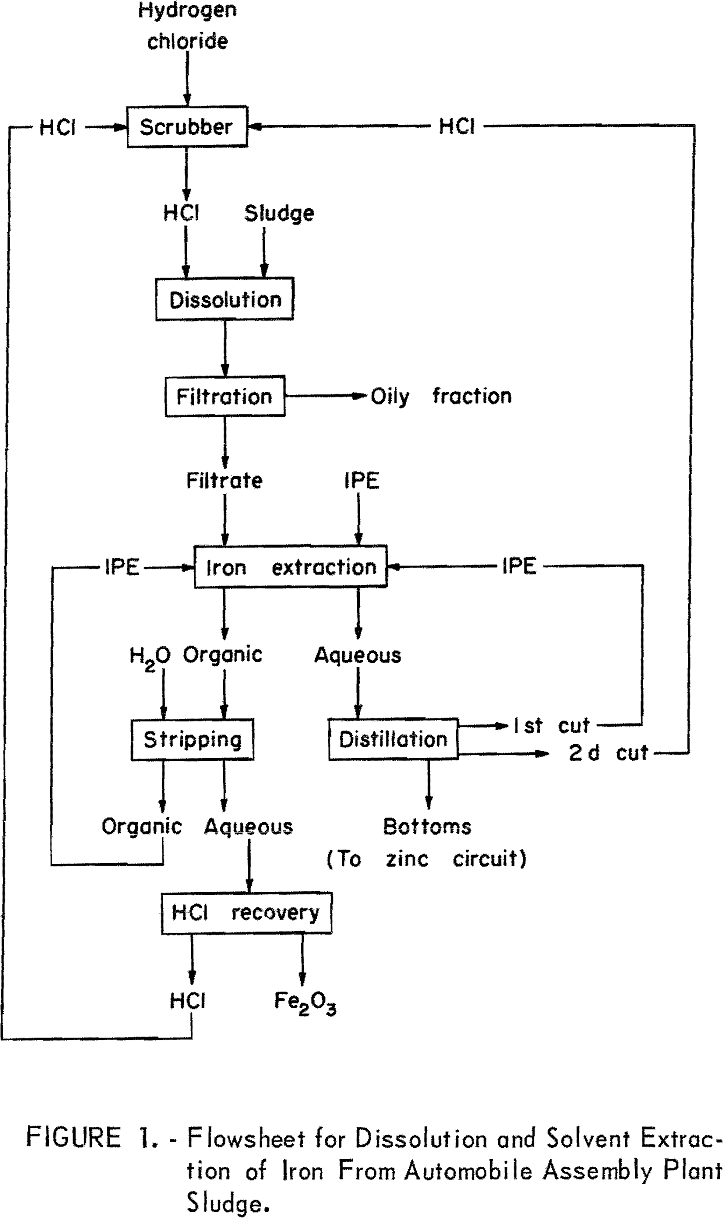
Two liters of water was added to the loaded IPE in the mixer-settler, and the mixture was agitated about 15 minutes. Phase separation was rapid, and the aqueous ferric chloride solution (about 2.2 liters) was withdrawn. The regenerated IPE phase was recycled to the next iron extraction. The aqueous ferric chloride solution had a composition that resembled hydrochloric acid wastes from the pickling of steel, and could be treated by one of several industrial processes (1) developed to recover hydrochloric acid for recycling and iron oxide from hydrochloric acid pickling wastes. As an alternative procedure, the aqueous ferric chloride solution could be distilled to recover hydrochloric acid, and the ferric chloride residue could be disposed of by injection into deep wells.
The iron-free aqueous phase from the iron extraction (about 2-½ liters) was also distilled for the recovery of hydrochloric acid. The first low-temperature distillate (or cut) recovered the small amount of dissolved IPE, which was recycled to the next iron extraction. The second cut, which is constant-boiling hydrochloric acid, would be recycled to the scrubber for enrichment with hydrogen chloride (gas). The still bottoms from this distillation went to the zinc-extraction circuit.
The flowsheet for the zinc-extraction step is shown in figure 2. The aqueous phase from the iron extraction was treated with 79 grams NaOH to raise the pH to 3.5, which is optimum for the extraction of zinc using EHPA. The aqueous phase (2.5 liters) was agitated with – 5 liters of the organic phase for 15 minutes. After separation of phases, the aqueous phase (raffinate) was
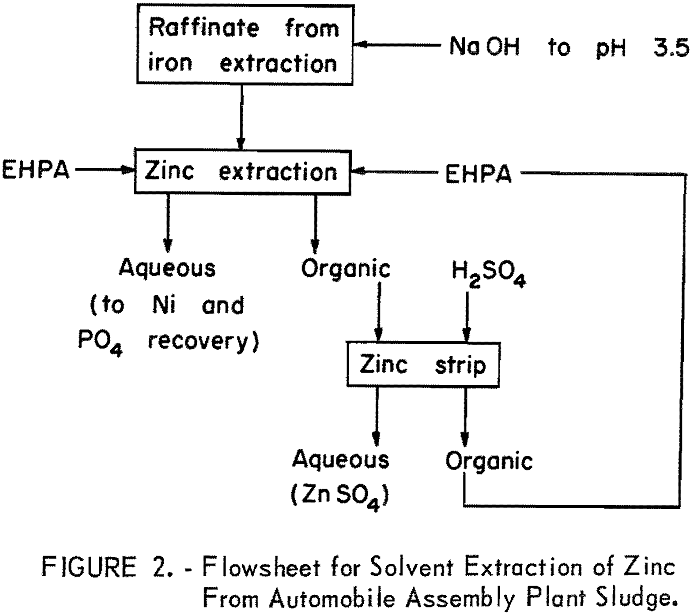
withdrawn and held for recovery of phosphates. The loaded organic phase was stripped in the same vessel by 1 liter of 15-percent sulfuric acid for recovery of zinc and regeneration of the organic phase. In practice, zinc sulfate would be recovered by partial evaporation of the sulfuric acid strip solution, cooling, and filtering off the zinc sulfate. The acid filtrate would be recycled to the next zinc strip.
The results of the sludge dissolution and the extraction of iron and zinc are summarized in table 2. Virtually all (99.7 percent) of the iron was extracted in the IPE in one stage, and essentially all the zinc and nickel remained with the aqueous phase. About 13 percent of the phosphate was extracted with the iron and the balance stayed in the aqueous phase. More than 98 percent of the zinc was extracted by the EHPA in one stage, and it was essentially free of other materials. Eighty-seven percent of the original phosphate content of the sludge remained in the raffinate. Very little of the metals or phosphates was found in the oily fraction.

Trisodium Phosphate Product
The raffinate from the zinc extraction was treated with sodium hydroxide until the pH was about 8.5 to precipitate the nickel as Ni3(PO4)2·8H2O, which was filtered off. When a sufficient amount of this nickel salt has accumulated, it could be further refined or sold to a chemical processing company. Additional sodium hydroxide was then added until the pH was about 11.5 to form trisodium phosphate. Technical-grade trisodium phosphate was recovered either by evaporating the solution (fig. 3) or by adding ethyl alcohol (fig. 4). If desired, disodium phosphate could be recovered at about pH 8.5 by the same methods.

In a typical sodium phosphate product, the only impurities detected by spectrographic analysis were calcium (0.06 percent) and manganese (0.007 percent). The lead content was less than 0.001 percent. X-ray diffraction analysis showed that the hydrated product was trisodium phosphate (Na3PO4·12H2O). After removal of the water of hydration by heating, the product contained the following, in percent: sodium, 43; phosphate, 53; and chlorine, 2.6. The theoretical content of sodium in pure trisodium phosphate is 42.1 percent.
The recovery of ethyl alcohol (if used) and sodium hydroxide from the sodium phosphate mother liquor by distillation and evaporation, respectively, would be economically desirable and would avoid the production of new wastes.
Appliance Sludge
Dissolution and Iron Extraction
In a typical test, 1,000 grams of filtered sludge, containing 700 grams of solids, was dissolved in hot, concentrated hydrochloric acid, and the oily material was removed as previously described. After the acid strength was brought up to 8 normal, the volume of the solution was 4 liters. Ten liters of IPE was used for the iron extraction. The flowsheet for the dissolution
and iron extraction was identical to that for the automobile sludge (fig. 1) except for two changes: (1) Much of the dissolved iron was initially in the ferrous state; the iron was oxidized to the ferric state by adding chlorine so that the iron could be extracted by IPE in the next step; (2) a two-stage water strip of the loaded IPE phase was employed (each strip was 2 liters).
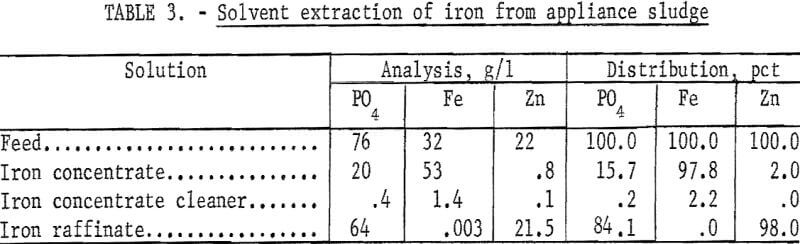
The results are summarized in table 3. Nearly 100 percent of the iron and about 16 percent of the phosphate were extracted, and 98 percent of the zinc remained in the raffinate. The results suggest that a one-stage water strip of about 2-½ or 3 liters would have been adequate to remove essentially all of the iron. The material balance is for the iron extraction only.
Zinc Extraction
The flowsheet for the zinc extraction was the same as for the automobile sludge (fig. 2) except that two extraction stages were used (sequence: extraction, strip, extraction, strip). About 4 liters of the aqueous iron raffinate was extracted by 8 liters of the organic phase, and two 1-liter acid strips were used. The. results are given in table 4. Almost all of the zinc was extracted; the phosphate remained in the raffinate. The zinc concentration in the iron raffinate was about three times as much as that in the work on the automobile sludge; the efficiency of zinc, extraction was nearly 100 percent. The results also indicate that the volumes of both the organic phase and the acid strip in the second extraction stage could be greatly reduced, which would decrease the amount of phosphate extracted. The material balance is for the zinc extraction only. The overall recovery of zinc was 98.0 percent, and of iron, nearly 100 percent. The zinc raffinate contained 83.6 percent of the original phosphate content of the sludge.

The raffinate from the zinc extraction was treated with sodium hydroxide until the pH was about 8 to precipitate cobalt and nickel, which were filtered off. Trisodium phosphate was recovered by the same procedure described earlier.
Discussion
The described recovery process has two drawbacks: The process is complex, and the IPE extracted some phosphoric acid along with the ferric chloride. However, the extraction parameters were not optimized in this work, and finding the optimum conditions would improve the process. The data on the iron extraction indicate that ferric chloride is much more readily extracted by IPE than is phosphoric acid; therefore, reducing the volume of IPE in the extraction should significantly decrease the amount of phosphoric acid extracted (by the salting-out effect). The data also indicate that the volume of the organic phase used in the zinc extractions could be significantly reduced.
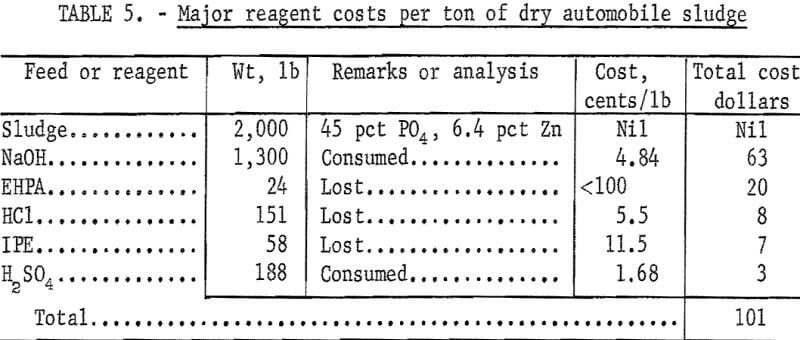
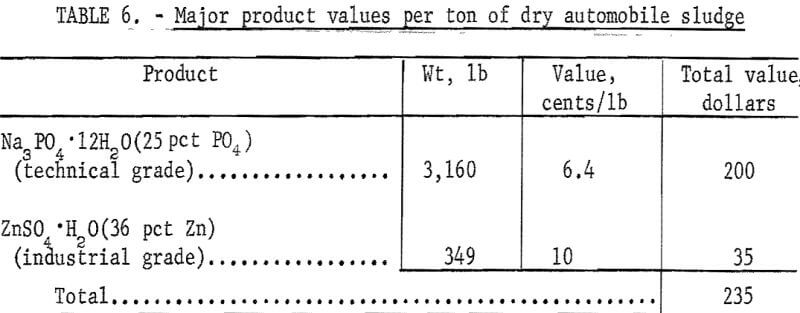
Further and larger scale research would be necessary to accurately determine the economics of the described process. However, the approximate values of the principal reagent consumptions and of the major products per ton of dry automobile sludge were calculated (using prices in the November 1, 1971, issue of the Oil, Paint and Drug Reporter) and are given in tables 5 and 6, respectively. The value of two marketable, products totals $235 per ton, and the cost of the major reagents consumed totals $101 per ton. Kerosene losses were negligible, and a 5-percent loss of hydrochloric acid was assumed. Reagent costs would be reduced if soda ash (Na2CO3) were used for neutralizing the solutions up to pH 7, followed by the use of sodium hydroxide to produce trisodium phosphate.
Advantages of the process are that extraction efficiencies were high, and solvent losses were low. In closed industrial extraction apparatus, solvent losses should be even lower owing to reduced evaporation. If the waste phosphate sludges from many sources in an industrial metropolitan area were to be collected and processed in a central plant, significant savings would result from the larger scale of operations.
Conclusions
Waste phosphate sludges resulting from the manufacture of automobiles and appliances can be successfully treated by solvent extraction techniques to produce separate zinc, iron, and trisodium phosphate products. After dissolution in hydrochloric acid, iron was extracted as ferric chloride by isopropyl ether, and zinc was extracted by di-2-ethylhexyl phosphoric acid in kerosene. Zinc recoveries were about 98 percent, iron recoveries were nearly 100 percent, and 84 to 87 percent of the phosphate remained in the raffinate. Technical-grade trisodium phosphate was recovered from the raffinate. The efficiencies of the one- and two-stage zinc and iron extractions ranged from 98 to nearly 100 percent, and zinc and iron were readily stripped from the organic phases. Solvent losses were low.
