Metallurgical processes involving the use of cyanide such as gold cyanidation, metal plating and flotation, generate effluents containing cyanide and cyanide in complex combinations with heavy metals in quantities exceeding those regarded as being safe for discharge into streams.
A new, inexpensive method for removal of cyanide, combined cyanide and related species from wastewaters has been developed in Inco laboratories. The method is based on the use of sulfur dioxide (or a sulfite), lime and air as the reagents. The decomposition reactions of cyanide, combined cyanide and related species are catalyzed by copper in solution. Complete removal of cyanide species, most of which are oxidized to harmless cyanate, is obtained by treating copper containing cyanide effluents with sulfur dioxide-air mixtures while maintaining the pH between 6 and 10 with addition of lime.
Effect of Process Variables in the Removal of Cyanide and Related Species
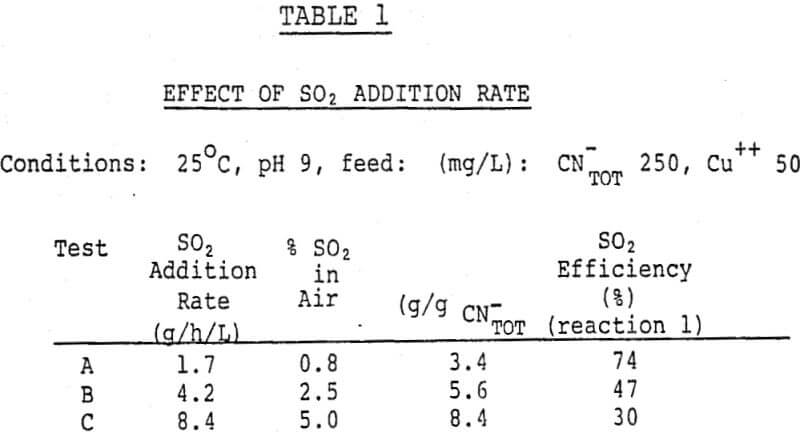
The effects of process variables were usually studied in batch experiments mostly using synthetic liquors. The tests were carried out using a 1 liter capacity stirred reactor, with addition of sulfur dioxide and air premixed at 0.5 to 5 volume percent sulfur dioxide at a rate of 1 liter of air per minute per liter. The pH of the reaction mixture was controlled by addition of a lime-water slurry (3.8% lime by weight) using a Radiometer titrator.
The effects of adding respectively 50 mg/L of Cu++, Ni++, Mn++, Co++, Zn++ (as sulfates) and Ag (as nitrate) to a solution containing 250 ppm cyanide as sodium cyanide, on the rate of cyanide decomposition with sulfur dioxide, air and lime at pH 9.
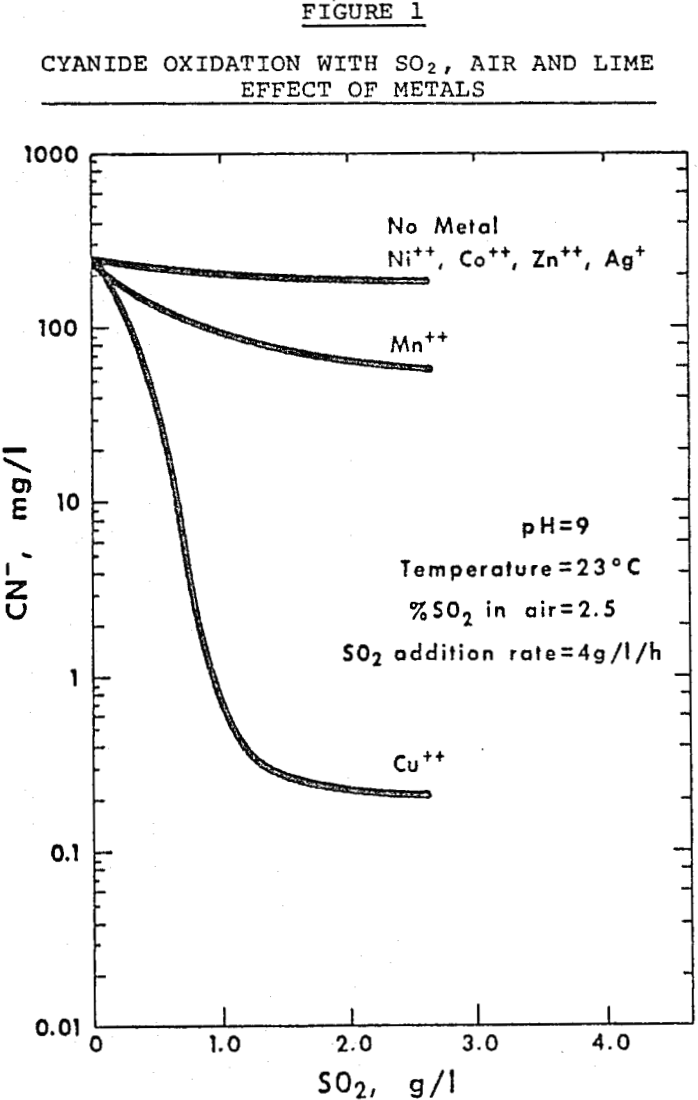
Temperature has no significant effect on the initial rate of cyanide decomposition in the range 5 to 60°C, but lower residual cyanide levels are obtained at high temperature.
The effects of adding respectively 50 mg/L of Cu+ (as chloride), Cu++, Ni++, Co++, Mn++ (as sulfate) and Ag+ (as nitrate) to a solution containing 150 ppm thiocyanate as sodium thiocyanate, on the rate of thiocyanate decomposition with sulfur dioxide, air and lime at pH 9 and room temperature.
In the presence of 50 mg/L Ni (added as sulfate), the effect of varying the treatment pH in the range 8 to 10 at room temperature is shown on Figure 6. The results indicate that the catalytic effect of nickel is best at pH 9 or above.
Although copper is a catalyst for the oxidation of cyanide and copper, nickel and cobalt are catalysts for the decomposition of thiocyanate with sulfur dioxide, air and lime at pH 9, some of the catalytic effect of these metals is lost when treating effluents containing combinations of cyanide and related species.
In effluents containing cyanide, thiocyanate and thiosulfate in the presence of copper, copper is a catalyst for cyanide oxidation and not for thiocyanate.
As will be shown in examples given further in the text, cyanide combined with zinc, cadmium and arsenic is also oxidized with sulfur dioxide, air and lime in the presence of copper. The preferential order of decomposition of the metal cyano-complexes is:
Zn (CN)4²- > Fe (CN)4²- > Ni (CN)4²- > Cu (CN)3²-
Cyanide and combined cyanide can also be oxidized with sulfur dioxide, air and lime in the presence of pyrrhotite at room temperature and in the pH range 8 to 9, provided a sufficient amount of copper is present or added to the pulp.
Treatment of Industrial Waste Waters
Flotation tailing liquors usually contain cyanide, thiocyanate and thiosalts in variable amounts and copper and nickel as major metal impurities. The tailings may also contain flotation agents (xanthate) and trace quantities of precious metals.
An example of a two stage continuous treatment of flotation tailings effluent liquor using synthetic stack gas and lime. The total cyanide was oxidized down to 0.6 mg/L with reagent consumptions of respectively 9.8 g of sulfur dioxide and 6.6 g of lime (CaO) per g of cyanide.

