Table of Contents
The Bureau of Mines strives continually toward its goals of maximizing minerals and metals recovery from primary and secondary domestic resources, and reducing energy requirements of mining and mineral processing. This investigation to improve zinc electrolysis by separating and recovering the currently discarded cobalt and nickel found in Missouri zinc concentrates is among the Bureau’s many research and development activities directed toward improving the recovery of critical minerals. Missouri zinc concentrates contain about 0.02 pct Co and 0.03 pct Ni, a potential resource that is now causing major problems in zinc electrowinning. The adverse effects of cobalt and nickel are great enough to limit the amount of Missouri zinc concentrate being processed by electrolytic-zinc producers.
Impurities such as cobalt and nickel in the electrolyte cause serious reductions in zinc electrowinning current efficiency at impurity concentrations of >10 ppm. The metals are deleterious to zinc electrolysis but are not codeposited to any appreciable extent. Their action in the zinc electrolysis cell is probably one of being alternately deposited on the zinc surface and then dissolved by the sulfuric acid present. The presence of these impurities causes localized reduction of the hydrogen overvoltage and spot burning of the deposited zinc, and consumes power that would otherwise be used to deposit zinc.
The usual industrial technique for purifying zinc sulfate solution for zinc electrolysis is a two-stage operation. The solution is heated to 90° C, and then copper sulfate and arsenic trioxide are added, with coarse zinc (+65 mesh) being added shortly thereafter. Zinc dust is added in batches until the cobalt is <10 ppm. Once the cobalt has dropped to this level, the nickel is assumed to be less. Further purification by filtering, the addition of a fine zinc dust (-325 mesh), and cooling removes the cadmium. The two filter cakes are both salable products, the first for its copper content and the second for its cadmium content, with cobalt and nickel being discarded to the tailings. The filtered zinc solution is now ready for zinc electrolysis,
This process for purification of zinc electrolyte is complicated and depends on the presence of other metal ions, type and size of powdered zinc added, and temperature. In addition, cobalt and nickel are not recovered. However, techniques for the analysis of cobalt using α-nitroso-β-naphthol, described as early as 1885 by Ilinsky and Knorre and later by Saltzman and Stary, showed that cobalt could be extracted in the presence of ppm quantities of zinc and other metals. Similar analytical techniques for nickel analysis are described by Voter and Monnier. The objective of the work described in this Bureau of Mines report was the technical evaluation and adaptation of those analytical techniques for the selective recovery of cobalt and nickel from a 200-gpl-Zn (493-gpl-ZnSO4) solution to produce an industrial zinc electrolyte suitable for electrowinning.
Experimental Procedures
The zinc sulfate solutions for all tests were prepared by dissolving 249 gpl French-process zinc oxide (ZnO) in a 300-gpl sulfuric acid solution. The volume was adjusted to 2 liters with distilled water. Purification of the solution was necessary because of impurities contained in the zinc oxide. The purification was started by raising the pH to 5.4 by small additions of zinc oxide; the solution was then oxidized with 0.1 N KMnO4 for 2 hr, heated to a hard boil for 20 min, and filtered.
The filtrate was purified further with the addition of 2.5 gpl high-purity zinc dust (-65 mesh). The solution was stirred, brought to a vigorous boil for 20 min, and filtered as before. After cooling, the solution was poured into a 2-liter volumetric flask, and the volume was adjusted with distilled water. The electrolyte was stored in a glass bottle.
The cobalt and nickel additives were prepared as standard stock solutions of reagent-grade sulfates. The cobalt sulfate (CoSO4) powder required several hours to solubilize in distilled water (pH = 5.4). The nickel sulfate solubilized quickly. The solution concentrations were made such that 1 ml of the specific sulfate solution, when added to 1,000 ml of the prepared zinc sulfate solution, resulted in a 50-ppm concentration of cobalt (II) or nickel (II) ions.
Reagent-grade solvents and complexing reagents were used without further purification. The complexing reagents were dissolved in the appropriate solvents to the desired concentration. All solvent extraction tests were started by adding the organic phase to a 125-ml separatory funnel containing the zinc sulfate solution (cobalt and nickel added) at a specific pH. The two phases were shaken vigorously with a Burrell Wrist-Action Shaker for 15 to 30 min at room temperature. The immiscible phases were allowed to separate, and a 25-ml aqueous phase sample was taken for chemical analysis.
Precipitation experiments were initiated by adjusting the pH of the zinc sulfate solution with sulfuric acid and then heating to 30° to 55° C, depending on the precipitating reagent. The precipitating reagent was added either directly or predissolved in a minimal amount of ethanol before being added to the zinc sulfate solution. The solutions were agitated for a predetermined time, and the precipitate was filtered. The percent cobalt and/or nickel precipitated from the sulfate solution was determined by analysis of the solution before and after precipitation.
Results
Solvent Extraction
This investigation examined many reagents that complex with cobalt and/or nickel, but only a few gave the desired extraction. The most promising were α-nitroso-β-naphthol, β-nitroso-α-naphthol, 1,2-cyclohexane dione dioxime (nioxime), di-2-pyridyl ketone oxime, and dimethylglyoxime. Their chemical structures are shown in figure 1. All of the complexing reagents have the organic functional group of oximes (-NOH). Oximes are known to complex with many of the metal ions. They are the base for several commercially available solvent extraction reagents and ion exchange resins.
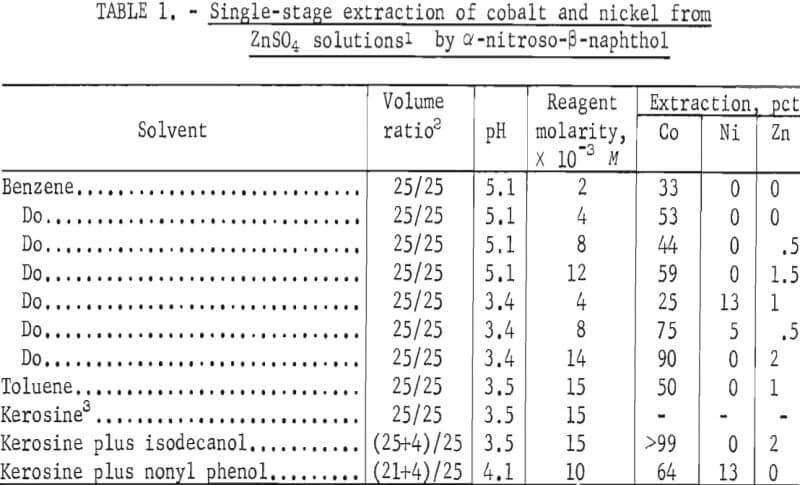
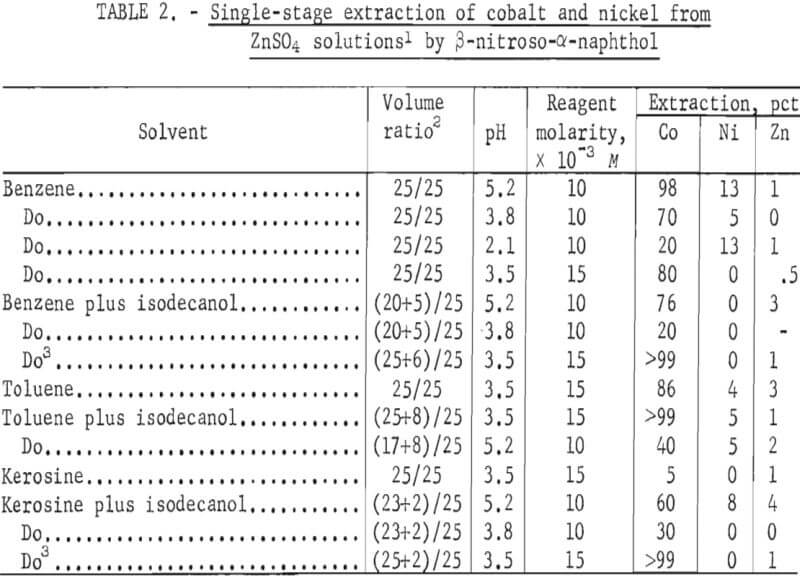
The results of tests with the reagents α-nitroso-β-naphthol and (β-nitroso-α- naphthol in various diluents, pH, and reagent concentrations are shown in tables 1 and 2, respectively. The data show that both reagents primarily remove cobalt and that extraction is dependent on pH, reagent concentration, and diluent. A maximum of 4 pct Zn was coextracted.
The use of α-nitroso- β-naphthol resulted in the extraction of >99 pct of the cobalt at a pH of 3.5 in the diluent kerosine-isodecanol. Ninety percent of the cobalt and essentially none of the nickel was extracted at a pH of 3.4 in benzene.
β-Nitroso-α-naphthol in various diluents gave results similar to those with α-nitroso-β-naphthol. Good extraction was obtained in the diluents benzene, toluence, and kerosine. The addition of isodecanol increased the cobalt extraction in most cases. The best extraction occurred at pH of 3.5 and reagent concentration of 15×10-³.
The primary difficulty in using either of these reagents is that the cobalt cannot be stripped from the organic phase because it is oxidized to cobalt (III). The cobalt-nitrosonaphthate is very stable and does not release the cobalt during the stripping stage. However, both are relatively inexpensive.
Data from tests utilizing 1,2-cyclohexane dione dioxime (nioxime) are shown in table 3. Greater than 99 pct of the nickel and 75 to 80 pct of the cobalt were extracted at a pH of 5.2 in the diluents toluene-isodecanol and kerosine-isodecanol. A pH <3.2 resulted in 99-pct extraction of nickel without the coextraction of cobalt. These results allow the nickel to be extracted at a pH <3 and cobalt to be extracted at a pH of 5 using a single complexing reagent. As much as 7 pct of the zinc was extracted in the toluene- isodecanol diluent. Zinc extraction increased with decreasing amounts of isodecanol. Essentially no zinc was extracted when the kerosine-isodecanol diluent was used.

Stripping test data with sulfuric acid reveal that the metals can be stripped from the loaded nioxime organic phase as shown in table 4. The use of high acid concentration strips the metals but does not regenerate the nioxime functional group. Further contact of the stripped nioxime organic phase with fresh aqueous feed does not result in further extraction of cobalt and nickel. Further testing is required to develop a technique to recycle the nioxime. Nioxime has been recovered from hydrochloric acid, but little work has been done in a sulfate medium.

The reagent di-2-pyridyl ketone oxime in chloroform extracted 96 pet of the cobalt. The data are shown in table 5. Of the diluents tested, chloro-form was the only one that gave the desired results. Further work was discontinued because of the possible hazards of working with chloroform. Many other solvent extraction systems proved to be ineffective.

Precipitation
α-Nitroso-β-naphthol (in the form of sodium naphthenate and sodium nitrite) has been used to precipitate cobalt from zinc sulfate solutions commercially. Zinc electrolytic plants in Australia make use of this technique. The precipitated cobalt-nitrosonaphthate is recovered and oxidized at elevated temperatures to a cobalt oxide.
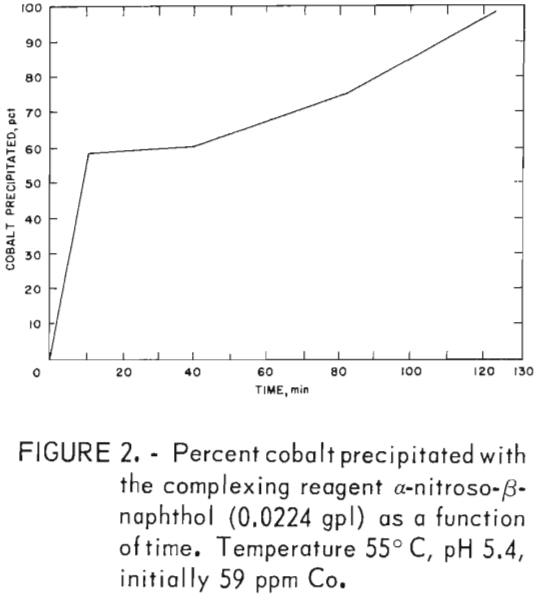
Precipitation data in table 6 were obtained by dissolving α-nitroso-β-naphthol in a minimal amount of ethanol and adding it to the prepared zinc sulfate solution. Ninety-seven to 99 pct of the cobalt was precipitated; a maximum of 20 pct of the nickel was coextracted at a weight ratio of 17.4 and over a pH range of 2 to 5. The stoichiometric weight ratio is 8.8:1, which is approximately half that required for the complete precipitation of the cobalt. Similar data were obtained when the reagent was added directly (without prior dissolution in ethanol) to the prepared zinc sulfate solution. Figure 2 data show that maximum cobalt precipitation occurred in 2 hr.
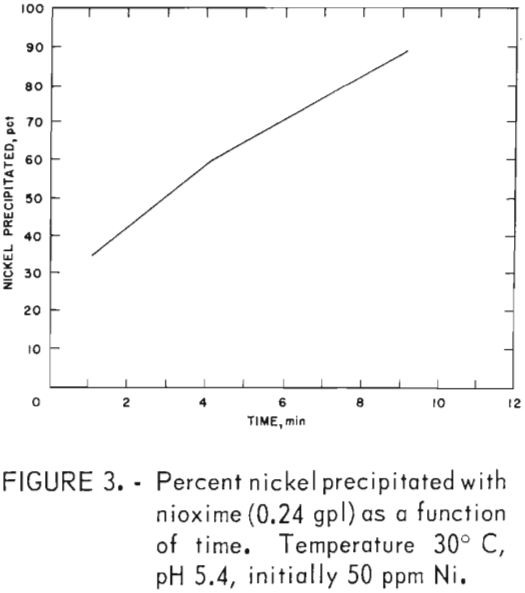
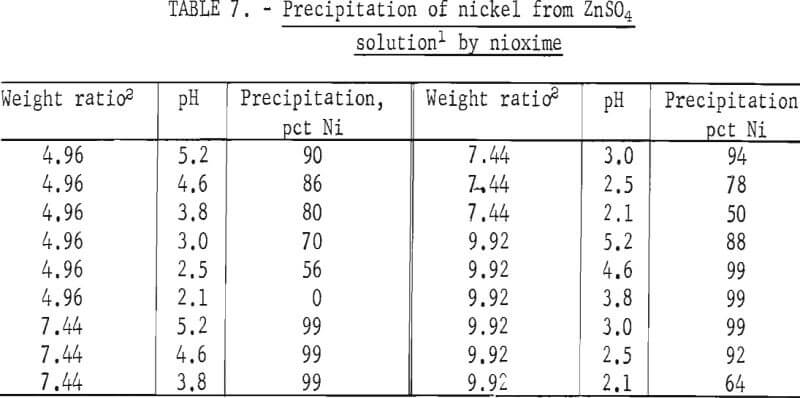
The direct addition of powdered nioxime to the nickel-bearing zinc sulfate solution resulted in precipitation of 99 pct of the nickel. These data are shown in table 7. Nickel precipitation occurred at a weight ratio between 5 to 10 and over a pH range between 3.0 to 5.2. At weight ratios of 4.96 and 7.44, 90 and 99 pct, respectively, of the nickel was precipitated. The stoichiometric weight ratio is 5.8. Figure 3 shows that nioxime precipitates all of the nickel in 10.5 min.

The addition of dimethyglyoxime dissolved in a minimal amount of alcohol precipitated 84 pct of the nickel from the prepared zinc sulfate solutions, as shown in table 8. Maximum precipitation occurred at pH 4. Further decrease in pH resulted in increased coprecipitation of zinc. The reagent precipitated the nickel in less than 15 min. Cobalt was not coprecipitated at any pH.

Electrolysis
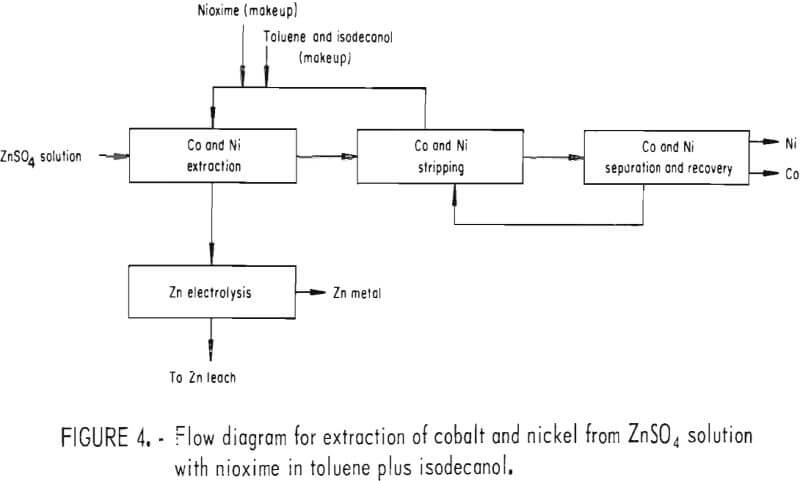
To fully evaluate some of these techniques for removing cobalt and nickel, electrowinning tests were conducted. The first series of tests used solvent extraction to purify the prepared zinc electrolyte as shown in figure 4. The organic phase contained nioxime (1×10-² M) in toluene (75 pct) and isodecanol (25 pct). An aqueous phase (200 gpl Zn, 50 ppm Co, and 50 ppm Ni) at pH = 5.2 and 25° C was added to the organic phase. The organic-aqueous phase ratio was 1.0, and the container was shaken for 15 min. The two phases were allowed to separate for 5 min.
The electrolysis cell (fig. 5) was a liter glass beaker fitted with a Teflon cover, thermometer, temperature-control probe, and magnetic stirrer
bar. An aluminum electrode holder held the platinum anodes (12×3.5 cm) and an aluminum cathode (10×3 cm) 4 cm apart. Polypropylene edge strips were used on the cathode to aid in stripping the zinc deposit. The cell was run at 860 A/m² (80 A/ft³) at 32° to 45° C. The cell solution concentration was adjusted by acid or distilled water additions at the beginning of the test. No further concentration adjustment was made during the batch test. The concentrations were changed to determine effects of acid and metal concentration on the deposits. The data in table 9 and the picture of the deposits in figure 6 show that a good smooth zinc deposit can be produced.



The second electrolysis series used precipitation techniques to purify the zinc electrolyte according to the flowsheet shown in figure 7. The zinc solution (pH 5.0) containing cobalt and nickel was heated to 55° C, α-nitroso-β-naphthol was added directly, and the solution was agitated for 2 hr. The cobalt precipitate formed rapidly and filtered easily. Nioxime was added to the warm filtrate, which was then agitated for 10 min and filtered again. The new filtrate stood overnight, during which time more Ni-nioxime precipitate formed. After another filtering, the solution was ready for electrolysis. The cell solution concentration was adjusted as described above, and the tests were run in the same manner. The cell was operated at 893 A/m² (83 A/ft²) to 947 A/m² (88 A/ft²) and 32° to 45° C. The conditions and a description of the zinc deposits are given in table 10, while the actual deposits are shown in figure 8. A good deposit was produced from a ZnSO4 solution which still contained 8 ppm Co and 3 ppm Ni.



Conclusions
The data from this Bureau of Mines investigation show that cobalt and nickel can be removed from a prepared 200-gpl-Zn electrolyte by solvent extraction or precipitation. Cobalt was extracted to <0.1 ppm from the prepared zinc sulfate solution by solvent extraction with α-nitroso-β-naphthol in benzene, kerosine-isodecanol, and toluene. β-Nitroso-α-naphthol in similar diluents was also effective. Cobalt and nickel were extracted to <0.1 ppm in a single contact with 1,2-cyclohexane dione dioxime and di-2-pyridyl ketone oxime. Stripping the complexed reagents from the solvents, with the exception of 1,2-cyclohexane dione dioxime, was not possible; however, the relatively inexpensive nature of the reagents makes their use feasible.
The direct addition of powdered α-nitroso-β-naphthol or 1,2-cyclohexane dione dioxime to the prepared zinc sulfate solution precipitated the cobalt and nickel collectively or individually, depending on the pH.
Electrolysis of the prepared zinc sulfate solution purified by these techniques produced good smooth zinc deposits. The current efficiencies were less than that required for industrial standards; therefore, optimization of the electrowinning procedure is required.

