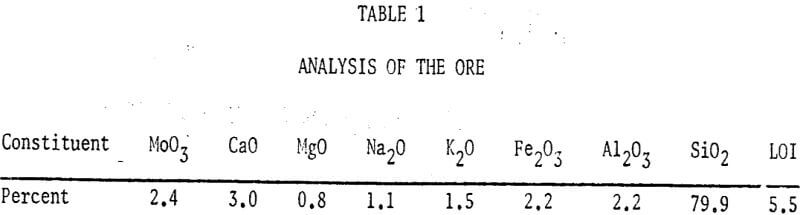
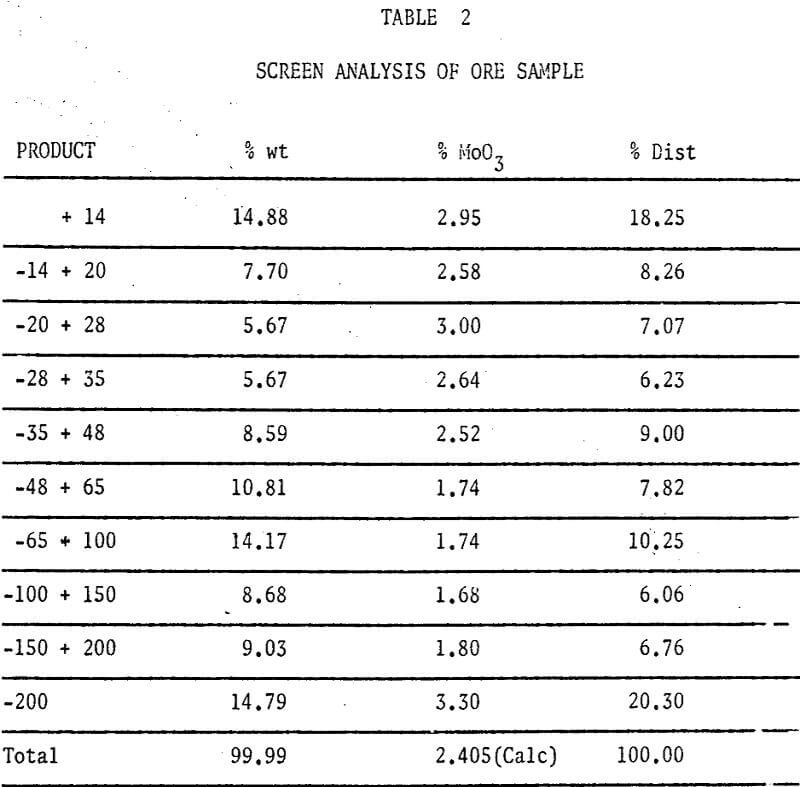
A study of some variables affecting the recovery of molybdenum from oxidized molybdenum minerals was made. The effects of variations in contact time, temperature, pulp density, particle size, and solvent concentration on the recovery of molybdenum using acid and alkaline solvents were investigated. Chemical analyses, screen analyses and microscopic examinations were made and the presence of important elements determined.
Description of the Ore
Geologically, this ore occurs in the bottom of the Chadron formation sandstone at the disconformity between the Chadron formation and the Pierre shale. Hand samples of the mineral ilsemannite have also been recovered from this deposit. The rock is composed predominantly of silica sand. The bonding material contains the molybdenum minerals and the iron minerals hydrated and otherwise.
The ore sample was stage crushed through 6 mesh and mixed by recombining splits from a Jones riffle. Upon further sampling and analysis it developed that we could cut reproducible (± 0.01% M0O3) samples at 100 gms wt from our minus 6 mesh aggregate.
Experimental Work.
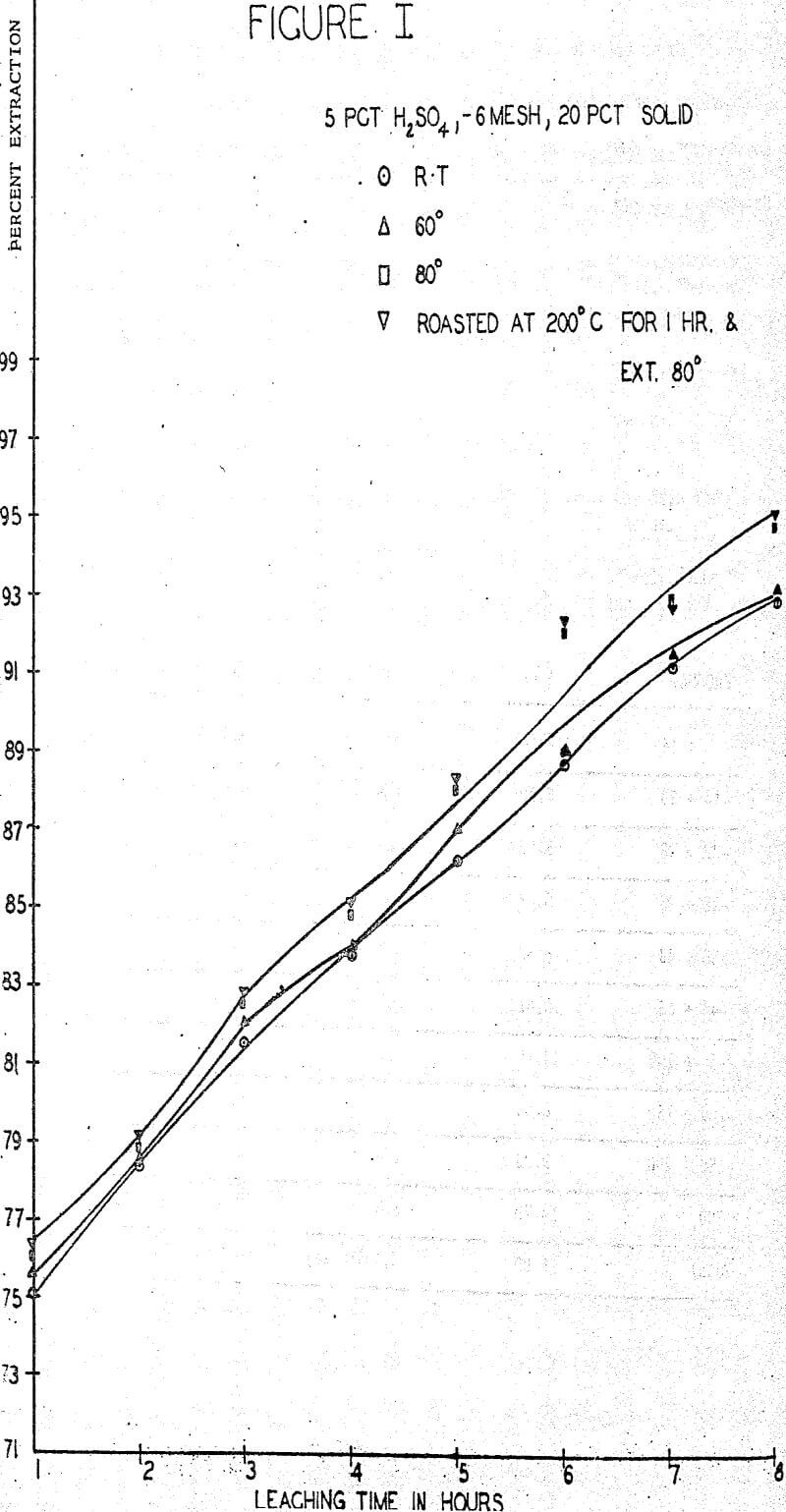
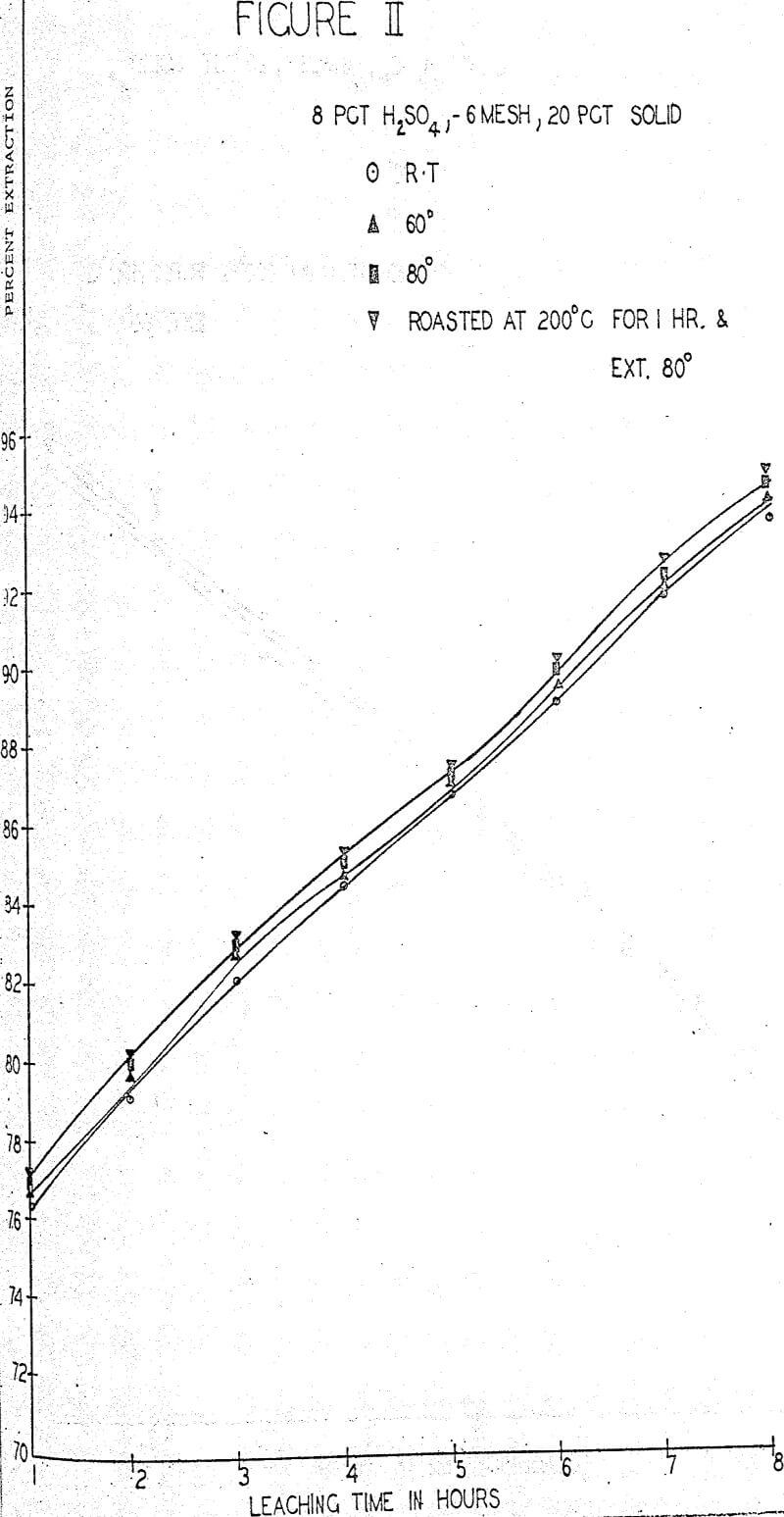
The first sequence of tests were run to determine the effect of roasting on molybdenum extraction. A series of samples was roasted for one hour at temperatures of 100°, 200°, 300° and 400° C in air in an electric furnace but no significant changes in extraction or filterability were noted. One large batch of ore was roasted for one hour at 200° C and used for parallel tests whenever filtration difficulties were anticipated. About 0.1% increase in extraction was obtained rather consistently from the roasted ore.
A standard leaching procedure was adopted for these tests. One Kg of ore was placed in a four liter beaker and leached for eight hours. Samples were taken every hour and the filtered liquid was analyzed for molybdenum. The pulp was agitated with an electric stirrer for the whole period with sufficient vigor to keep all solids suspended. The remaining pulp was filtered, washed and the solids and liquids were analyzed for molybdenum.
Recovery of Molybdenum from Solution
The molybdenum was recovered from solution in three ways. It was precipitated by milk of lime, adsorbed on activated charcoal and removed from solution by solvent extraction.
This was a relatively easy way to remove molybdenum from solution. When a moderate excess of lime was used, quantitative precipitation resulted. However, this method was not very selective. The suspended solids and other soluble constituents tended to dilute the precipitate.
A solution from a leach for eight hours with 5% acid, was agitated with activated charcoal for six hours. Liquid samples were taken and tested for molybdenum every hour until only a trace remained. The charcoal was then removed and washed and stripped with a 10% NaOH solution. The recovery of Mo in the caustic solution was 93.3% which was, within limits of error, the same as the extraction.
The work done in this field was preliminary by nature. A sample of leach solution was shaken by hand for one minute with an organic solvent in a separatory funnel and then allowed to stand four minutes for phase separation. The leach liquor was contacted successively with fresh organic solvent rather than simulating the counter current principle.
Three solvents were used. Each was a 10% solution of an amine in kerosene. The effectiveness of these amines to form organic soluble complexes was pH dependent. For the acid range, pH is less than two, all three collected more than 99% of the molybdenum into the organic phase in 10 or 11 contact cycles. Two amines, 9D-178 and S-24, were less effective at pH of 4. The other one, Aliquat-336 (a quaternary amine), was less sensitive to changes in acidity.