Table of Contents
- Prevent Gold Losts by Dusting
- Effect of Adding Nitre Cake Direct
- Effect of Temperature in the Nitre Cake Method
- Time Required for the Nitre Cake Method
- Vessel Required for the Nitre Cake Method
- Limitation to the Use of Iron Vessels
- Dry Methods of Refining Gold Slimes
- Slimes from Electrolytic Refining of Copper
- How to Separate Gold from Platinum and Iridium
- Purification of Bars of Base Bullion by Means of Sulphur and Soda
When carrying out experiments on the best methods of cleaning up or obtaining bars of bullion from gold slimes several points have to be considered. Large losses sometimes occur in ordinary practice in the drying and roasting of such finely divided material; losses occur through the feeding of such fine dust into the melting pots, and losses occur through gases and volatile products carrying with them finely divided gold when in the pots, and finally gold enters the slags. Independent of these losses is the bullion produced—as a rule it contains metals which were present in the slimes, such as arsenic, antimony, lead, zinc, copper, iron, tellurium, and selenium—sometimes one or other of these is absent, but it is rare to find perfectly clean bullion. Sulphur also forms a matte which sometimes encrusts the bar, or dissolves in the slag, carrying gold and silver with it.
Prevent Gold Losts by Dusting
Experiments were directed to discover a method which would avoid the losses by dusting and volatilization, and which would either produce a high grade bullion or else give fine gold.
The fact that the gold and other metals were in an extremely fine state of division led to tests being made with solutions in order to see whether the base metals and silver could not be dissolved out in the wet way. The common solvents, such as nitric acid and sulphuric acid, proved ineffective on so many samples that it was evident that oxidation did not proceed at a high enough temperature: to remedy this, the slimes were moistened with sulphuric acid and then evaporated to dryness, and heated strongly. This served to effect the oxidation of nearly all the metals, and rendered part of the silver soluble. The gold, however, was left in such a fine state of division as to make it a difficult matter to handle it without loss, and even when more strong sulphuric acid was added and heated it was not found possible to remove the whole of the silver.
The slimes were next moistened with potassium bi-sulphate and dried, more bi-sulphate was added, and the mixture was strongly heated; after cooling, the mass was washed. The result was eminently successful. The bi-sulphate effected oxidation and decomposition of organic matter, cyanogen compounds, and transformed the whole of the silver present into sulphate.
On washing the mass with water the gold could be seen as spongy lumps, which could be washed readily. The gathering together of the gold is an interesting process, and is not due simply to the melting of the enclosing salts, for when other salts which melt at a red heat are used there is no tendency for the gold particles to adhere to each other until the temperature reaches a point near the melting point of gold. In the case of the bisulphates of the alkalies, when the slimes in contact with them reach a dull red heat large bubbles or blisters form, and the films forming the skin of the bubble appear to be pure gold. These films contract into wrinkled masses when the bubble breaks, and cohere together in sponge-like form. It would almost appear as if the gold at a certain stage passed into solution, and formed a fusible salt, which decomposed almost at the instant of its formation. Tests were made to determine whether gold was in solution at any time, but either no gold, or only traces were found.
When slimes are rich in gold, or contain 50 per cent, or more of that metal, the bubbles described do not appear, but the whole of the gold will, after fusion and washing, hang together in one coherent cake. If a large amount of inert material, such as calcium sulphate or silica is present, then a portion of the gold does not form coherent masses, but remains apparently attached to these individual grains of inert materials, giving a pink powder. The same coloured coating will be left in a porcelain dish if chloride of gold is heated with either bisulphate of sodium or sulphuric acid. It adheres so strongly that it can not be removed mechanically.
The gold left behind after the silver and soluble sulphates have been washed out, is, after smelting, almost pure.
Laboratory tests were universally so promising that operations were conducted on a larger scale. The cheaper compound bisulphate of sodium was first used, and finally nitre cake, or a bye-product from the manufacture of nitric acid. In manufacturing nitric acid excess of sulphuric acid is added to sodium nitrate in order that the temperature at which nitric acid is driven out may be so low that the acid is not split up into oxygen and nitric peroxide, also so that the resulting product left in the retort will melt at such a low temperature as to be readily run out.
If sulphuric acid is added according to the equation H2SO4 + 2NaNO3 = Na2SO4 + HNO3 a large amount of nitric acid would be decomposed, and the resulting salt would only melt at a temperature over 800 degrees C. If the quantities added are in accordance with the equation H2SO4 + NaNO3 = HNaSO4 + HNO3 the evolution of nitric acid takes place at a low temperature, and the bisulphate of sodium remaining in the retort is so liquid that it can readily be run out. The average sample used contained 36 per cent, by weight of acid, reckoned as sulphuric acid. Nitre cake is a product which is usually intermediate between these two, or an admixture of the normal sulphate and bisulphate. When bisulphate of sodium is heated it loses water, and is converted into pyro-sulphate.
2HNaSO4 = Na2S2O7 + H2O
The pyro-sulphate is a powerful oxidising and sulphating salt. It stands a dull red heat without decomposition, and at this temperature will attack and dissolve compounds not affected by acids which are volatile before this temperature is reached. At a full red heat it splits up into sulphate of sodium, and evolves sulphur trioxide.
Na2S2O7 =NaSO4 + SO3
The action of pyro-sulphate of sodium of silver may be represented by the following equation:
2Na2S2O7 +2Ag2 = Ag2SO4 + Na2SO4 + SO2
In presence of an oxidising agent the action is:
2Na2S2O7 +2Ag2 + O2 = 2Ag2SO4 + 2Na2SO4.
Silver, therefore, requires nearly double its weight when no oxidising agent is present, or its own weight when oxygen is supplied to transform it into sulphate.
Zinc, copper and iron, if decomposed in the same manner, require from two to four times their weight of the salt. As a matter of fact, it is necessary in practice to add more than the theoretical amount, since it is desirable to keep the mass molten so as to give every particle of slimes an opportunity for coming in contact with the solvent. A clean precipitate containing, for example, 50 per cent, gold, 10 per cent, of silver, and 10 per cent, of other metals, will require at least its own weight of nitre cake; most slimes are not so rich as this, so that usually double their weight is necessary. The cost of the nitre cake being only 30s. per ton, is so low in proportion to the value of the product that an excess can be used without sensibly increasing the cost of the operation. A few preliminary trials carried out in porcelain dishes with weighed quantities of the slimes and nitre cake will supply the information needed in a few minutes.
Effect of Adding Nitre Cake Direct
When nitre cake is added to large quantities of dried slimes the latter are not readily moistened or wetted by the product when it melts, the result is that dry patches of slime are left, and these may be blown out by the gases evolved on heating; further, if the nitre cake contains much water this is evolved, and much frothing takes place, causing the contents of the vessels to overflow. In order to avoid these two difficulties the slimes if dry were moistened with a small amount of aqueous sulphuric acid saturated with nitre cake, or really a solution of bisulphate of sodium in a dilute solution of sulphuric acid. The mixture was made into an almost dry mass. This was then placed in an iron vessel and heated to redness; there is no danger of loss by dusting, since the water is expelled quietly and the bisulphate on drying forms a cementing material which causes the slimes to bake into brown cakes; some of the base metals, as well as silver, are converted into sulphates, the organic salts, ferro and sulpho-cyanides, as well as sulphides are oxidised.
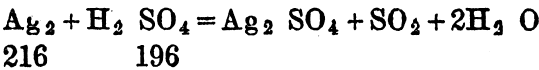
If 90 per cent, sulphuric acid is used the weight required will be approximately equal to the silver present.
As a matter of fact the other metals present are first converted into sulphates, and after the preliminary fusion it is found that only part of the silver will dissolve in water, and the gold remains still as a fine brown powder, difficult to handle. As soon as the baking operation has ceased, and the mass is dry and at a dull red heat, the nitre cake is added in lumps—usually it contains an equivalent of from 30 to 40 per cent, of sulphuric acid; it melts and appears to dissolve the baked slimes. If kept at a low red heat very little gas is evolved, but at a full red heat the pyro-sulphate is decomposed, and sulphur trioxide is abundantly evolved. While a small amount of nitre cake suffices to sulphatize the metals remaining, enough must be added to become molten; in that way the gold will collect and run together. The temperature also may be raised to a higher point with safety since sulphate of silver does not decompose while pyrosulphate of sodium is present. If a large excess of the pyrosulphate is used, and the mass kept at a dull red heat for some time, the gold will collect and fall to the bottom of the vessel, the upper layers containing very little. The silver sulphate appears to dissolve in the molten bisulphate since it remains diffused through it at the close of the operation.
Effect of Temperature in the Nitre Cake Method
It is necessary for the success of the process that the temperature be a full red heat throughout. It is only at that temperature that the gold will run together well, and that perfect decomposition of the silver salts can be effected. Chlorides, bromides, and sulpho-cyanides of silver are all decomposed and replaced by sulphate of silver. If the temperature becomes too high pyrosulphate of sodium is decomposed, and the less fusible sulphate of sodium remains, but so long as the fused product on being withdrawn shows a strongly acid reaction the temperature necessary to decompose sulphate of silver has not been reached. The sample can be withdrawn by means of an iron or clay rod, to which it readily adheres. Since the term red heat may convey a somewhat indefinite meaning, the correct temperature may be indicated as that which is attained in a muffle furnace when in proper working order, about one-fourth the distance from the front of the muffle. At a distance half-way in the muffle the pyrosulphate is totally decomposed, and part of the silver sulphate also; at the back of the muffle the temperature is sufficient to decompose the whole of the silver sulphate. Roughly speaking, the temperature will lie between 650 degrees and 800 degrees C. It is less than the melting point of sulphate of sodium. If too low a temperature be employed, a sample on being withdrawn cools down to a uniform brown mass, the brown colour being due to finely divided gold. If too high a temperature has been used the sample withdrawn on cooling is usually slate-coloured, due to the finely divided silver. When the correct temperature has been reached a sample, on cooling, will become bluish or greenish white, and small brown lumps of spongy gold will stand out from the surface.
Time Required for the Nitre Cake Method
When working with a few grammes the drying and fusion may be done in from 10 to 20 minutes; but when operating on large quantities it is a more difficult matter to heat the mixture. The preliminary drying and heating with a small amount of sulphuric acid and nitre cake may be done rapidly. The residual cake becomes heated very quickly. When the nitre cake is added in quantity it readily melts, and if heating is carried on too rapidly the portion exposed to the highest temperature will decompose before it has had time to react with the slimes; it is also difficult to get the heat to penetrate the mass, since while decomposition is going on the product is being cooled through the escape of water and gases; further, when large bubbles of gas form in such a molten mixture they appear to prevent the material adjoining them from rising in temperature. For these and other reasons the temperature should be gradually raised, and the mixture stirred to promote a more uniform temperature, and to counteract the effect of over-heating any part. The temperature is more easily regulated when an excess of nitre cake is used, for in this case the whole mass may be kept in a molten state for some time, and the liquid material poured out.
Vessel Required for the Nitre Cake Method
All preliminary tests were carried on in porcelain dishes or crucibles. These were unattacked, and gave satisfactory results. On a larger scale cast-iron pots were used, which were found to answer well when slimes rich in gold and poor in silver were treated. The pots were made from a close-grained white pig iron, those made from grey pig were too much attacked. A suitable pot for a hundred ounce trial is the well-known bowl-shaped, three-legged vessel known as a gipsy pot. This can be heated over an open fire or in a wind furnace having a small fire. The main objection to the use of the open fire is that the heating is more irregular, and that the fumes of sulphur dioxide evolved are very objectionable. If the pot stands in an assay or melting furnace the vessel becomes more uniformly heated, and the fumes escape into the flue.
After drying and the preliminary fusion, the nitre cake can be added gradually in lumps, the contents of the pot being well stirred. In a short time the lumps will melt and dissolve the fused cake. It is advisable to stir and heat until no lumps of the caked product can be felt. When fusion is complete the top layer may be poured off or ladled out on to a cold iron plate. If poured into water it will explode like matte, although it is probable that if the falling stream were struck by a jet of water it would granulate, and solution would take place much more speedily. The bottom layers can be ladled out, and, if necessary, be kept separate, since they contain the bulk of the gold.
Limitation to the Use of Iron Vessels
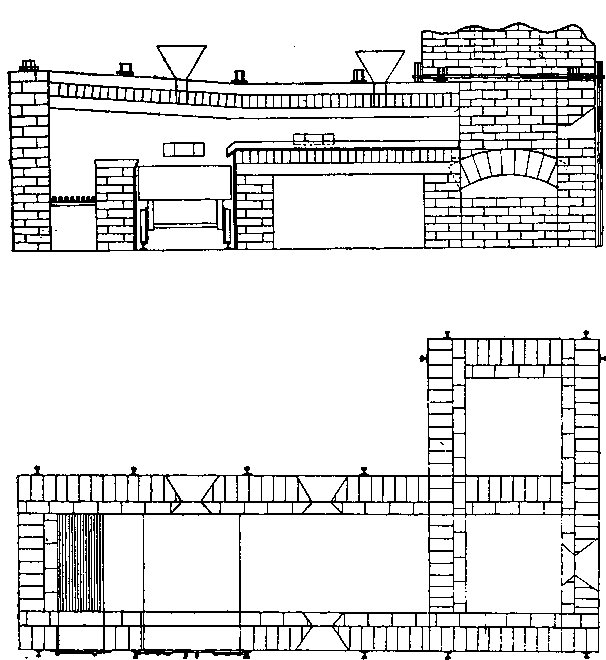
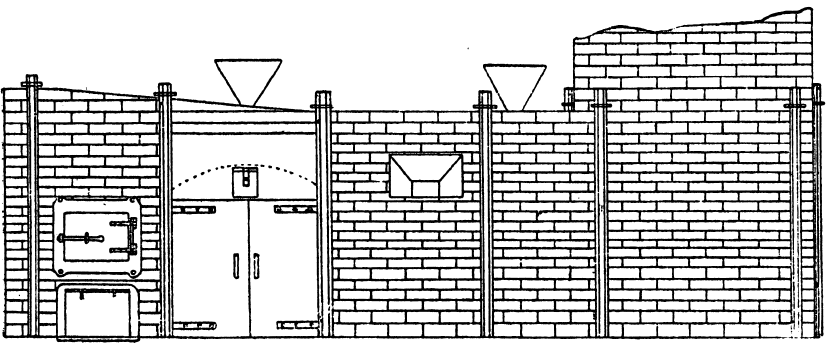
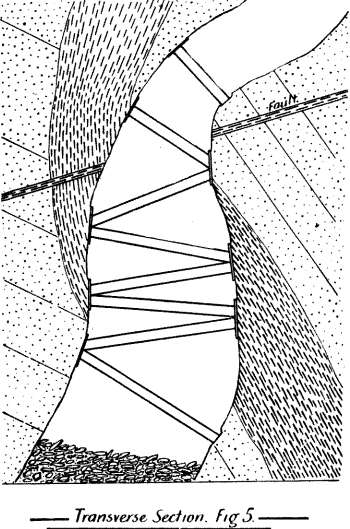
When molten silver sulphate comes in contact with metallic iron at a red heat the silver is reduced. If the amount of silver is small in the parcel then as the bulk of it as sulphate remains diffused through the molten cake, only a small proportion in contact with the iron is reduced, and even that quantity tends to pass into solution again, while pyrosulphate of sodium surrounds it. Should the sample carry a high proportion of silver then a protective lining must be given if an iron vessel be used. The best material is a silicious material which binds together well, and resists the action of the fused salts. Close-grained fire brick, fire tiles, or these set in fireclay, or even a good fire clay itself answers very well. Fireclay cemented with silicate of soda is satisfactory. Working scale tests indicated that it was desirable to heat the material by reflected heat from above so as to avoid troubles due to frothing and over-heating the vessel containing the slimes, as would probably be done by heating it below. A furnace was designed by the author, and subsequently erected at the Associated Gold Mines, Boulder City, W.A. It consisted essentially of a small reverberatory furnace, having a shallow pan for the hearth nearest the fireplace, and a flat plate of cast iron, with edges turned up further along. The pan was mounted on a truck, which could be run in and out on rails laid at right angles to- the longitudinal axis of the furnace, after the manner of the English Cupellation furnaces. The furnace itself was provided with a hopper for feeding nitre cake in, and also with two rabbling doors at the side, and one at the end. The object of the extended hearth covered by the iron plate was to provide a place readily heated where the preliminary fusion could take place. When this was finished the caked product could be worked down with rabbles into the pan where the additional nitre cake could be added, and the final fusion take place. The furnace so described acted well, but owing to other causes a test made on a large scale did not produce fine gold as anticipated.
Seeing with what rapidity the action takes place on a small scale in a muffle, where only thin layers of the mixture are treated, it would appear to be possible to make the operations continuous by allowing the molten material to pass through an orifice which is kept at the proper temperature, or even to flow down an inclined fireclay channel or pipe, heated to the required temperature.
https://www.911metallurgist.com/effect-various-impurities-gold-slimes-refining-process
Dry Methods of Refining Gold Slimes
The previous process, as described, entails a number of operations, which, although each is simple in itself, takes time, and requires more care than is often bestowed on such operations on mines. Efforts were made to achieve the same results in the dry way by means of one operation; these, though not wholly successful, give some instructive results. The first method attempted was based on the cementation process, in which the silver is transformed to, and remains as chloride, the base materials being fluxed out, and the whole melted down so as to obtain gold, chloride of silver, and a barren slag.
The slimes for this test were first treated with dilute sulphuric acid to dissolve the zinc, which was washed out as sulphate, the residual mass was moistened with just enough sulphuric acid to oxidise any organic matter, and convert the base metals into sulphates; then a small amount of nitre cake was added to enable the silver sulphate to stand a higher temperature without decomposition. The whole was then evaporated to dryness, and heated until the sulphur trioxide in excess was expelled. Sufficient salt was then added to convert the silver sulphate to chloride, and borax glass was added as a flux to remove bases such as iron, copper, as well as non-metallic compounds. This resulted in the production of borates, or borosilicates, and sulphate of sodium. When such a mixture was heated in clay pots, previously glazed by filling them with a saturated solution of borax, pouring this out, and then drying gradually, and heating strongly, the products separated in layers of chloride of silver, then a flesh-coloured layer of borosilicates. Covering this would be a white crystalline layer of sulphates and chlorides of sodium. When a large quantity of silver was present the chloride of silver formed a layer several times as large as the gold, but the gold in this case could not be brought up to more than 90 per cent. gold. In one case where the bullion, if smelted direct, gave a ratio of gold to silver of 1 to 12, or less than 8 per cent., it could be raised by this method to 87 per cent, gold—the rest of the silver passing into the form of chloride. Why the silver was not wholly converted is probably due to the access of some reducing agencies from the fuel or gases used for heating. If the slimes contained a relatively small proportion of silver, the gold produced would be nearly fine, but in no test was gold of greater fineness than 985 produced when operating on considerable quantities, which varied from ten to a hundred ounces. This method was abandoned when it was found on a small scale that gold was apt to volatilize owing to the production of chlorine, generated from the salt in conjunction with the oxidising salts present.
Refining Gold with Boric Acid
Borate of silver is not decomposed at a high temperature, and the next method adopted was based on that fact. The preliminary treatment of the slimes took place as before, and borax in sufficient quantities to form borate of silver and the base metals, was added, and the whole melted down. This gave a uniform slag into which the silver passed, and a layer of sulphate of sodium on top. The gold was cleaned of all base metals and most of the silver, but in no case was fine gold produced.
Caldecott’s Gold Refinement Method
In order to oxidise slimes more effectively during smelting, the use of manganese dioxide has been suggested. This substance has the advantage of parting with its oxygen at a fairly high temperature, while the manganous oxide produced forms a readily fusible silicate. It has also the advantage of being inexpensive. A better material is the crude manganate of potash or soda, which is an excellent oxidising agent in furnace operations, and owing to two bases being present, will form readily fusible slags. By using a sufficient quantity of this, sulphides, ferro-cyanides, and base metals may be oxidised, but the use of these materials is open to the objection that the bulk of the slag produced is increased.
Tavener’s Gold Refining Process (Smelting with Lead and Fluxes)
One of the best known methods of collecting, and at the same time purifying, gold and silver, is based upon the fact that molten lead readily dissolves those metals, and that any other oxidisable metals are oxidised and removed with litharge, which forms when the lead is oxidised. Although used on a large scale in metallurgical operations, and although it forms the basis of the dry assay of gold and silver, yet its application on a large scale to the treatment of auriferous slimes is due in South Africa to Tavener. The latest method of applying the process as described, appears to start with a preliminary acid treatment to remove the zinc (originally the slimes were dealt with direct). The cakes of slime, after leaving the clean up filter press, are placed in cast-iron pans, 3 feet by 2 feet by 5½ inches deep, and dried in a small reverberatory furnace; they are heated until all moisture is expelled—a white crust of zinc and calcium sulphate usually appears. The dried slimes are then sampled and assayed. As a working illustration of the process, a parcel of 22,996oz. was taken and found by assay to contain 3150oz. of gold. The lead considered sufficient is 10 times the weight of gold present, so 29,000oz. of litharge were mixed with the slimes and 3000oz. reserved for a cover. The flux used consisted of:—
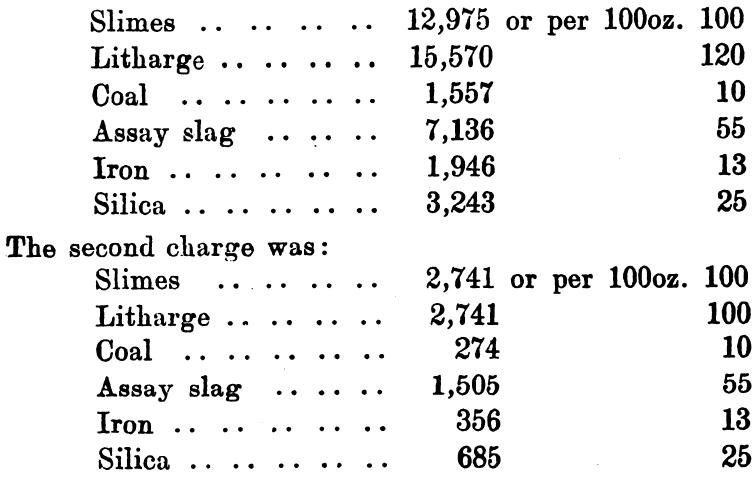
The first and second charges were melted together, and a cover of 3000 ounces of litharge added.
The third charge was:
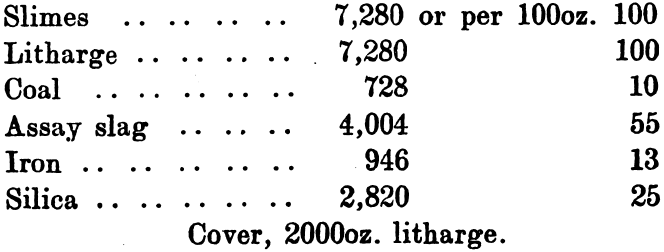
The iron used consisted of tin plate scrap cut into small pieces.
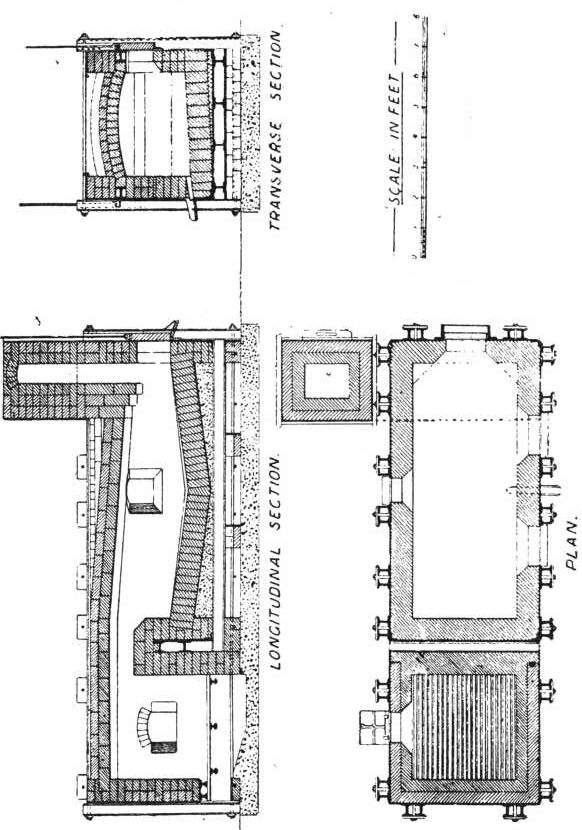
The charge was introduced into the furnace, five per cent, of the iron being added with the original flux, and the remaining eight per cent, added towards the close of the operation. After some hours the slag was rabbled off, the temperature being kept high to keep it fluid. Some of the slag contained much lead in the form of prills and had to be returned with the next charge. After about seven hours the lead was tapped out. The amount of lead bullion obtained was 28,204oz., containing 3166.3oz. gold. The excess of gold was said to be due to that in the slags from previous charges. The furnace used was a small reverberatory, the details of which are shown in the accompanying plans and sections.
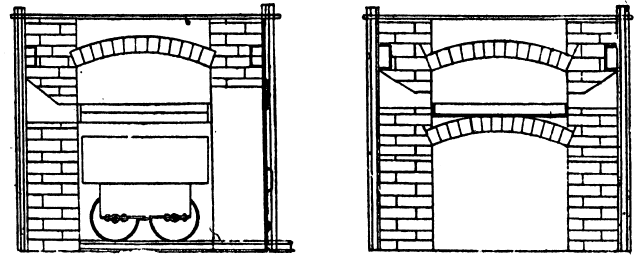
The lead bullion was afterwards treated on a cupelling furnace, constructed after the type of the old English cupel. The test is made from 200lb. of bone-ash and 14lb of caustic potash; this is moistened with 9 per cent, of water, and damped in layers into the test until it is full. The cupel is then hollowed out after the manner shown in section. A cupel will last at least over two cupellations. On firing the cupel is first gently, then strongly heated, the lead is carefully fed in at the back, and kept at a constant level. As oxidation proceeds the blast of air from the tuyere drives the litharge through a channel cut in the cupel, into a slag pot below. Towards the finish of the operation the cupel is allowed to absorb all the litharge produced. When nearly finished a flux composed of 10 borax, 5 soda carbonate, and 3 silica, is thrown on to the surface. The blast is again turned on and the molten slag run off into the pot. The gold, which is semi-solid but brittle at this temperature, is broken up with bars and melted in clay crucibles. A small amount of flux is added; this is thickened with bone-ash and skimmed off. The alloy produced from this assayed 858 to 861 fine, the balance being mainly silver.
The Tavener process supplies a means of dealing with large amounts of bullion in one operation, and when the gold is not contaminated with large quantities of base metals, such as copper, antimony, and arsenic, it should yield a bullion easily refined by cupellation. On the other hand, no operation with it is final, gold is left in the slags in the cupel, and other by-products which have to be re-treated. It does not go further than produce a gold-silver alloy. If used on raw zinc slimes or those from which the zinc has not been removed by a preliminary acid treatment, the litharge used would need to be eight times as great as the zinc oxide produced, since it takes that amount to dissolve it; the fluxes would therefore be excessive. It is also difficult to believe that no loss of gold takes place when zinc is oxidised in the air. Taking the results
as a whole they are no better than if the gold were melted in a reverberatory furnace, with suitable fluxes, in an oxidising flame, such as can be produced when using gaseous or oil fuel.
The Merril Process
A system introduced by Merril, at the Homes take Works, U.S.A., is somewhat similar to Taverner’s. The slimes are first treated with hydrochloric acid to dissolve the lime salts, then with sulphuric acid to dissolve out the zinc. The sediment remaining is then dried, mixed with litharge, crushed coke and borax, moistened with lead acetate, and briquetted under a pressure of from 2 to 3 tons per sq. inch. The briquettes are melted in an English cupellation furnace, the slags are drawn and cupellation continued in the same furnace.
Slimes from Electrolytic Refining of Copper
Not many samples of such slimes were available, and the only one on which tests were made came from the works of the Great Cobar Copper Company, N.S.W. In the electrolytic refining of copper, plates of impure copper are hung up as anodes in a bath containing sulphate of copper and some sulphuric acid. The current strength is so arranged that the copper is dissolved from the anode or impure sheets, leaving other metals unattacked, the silver, gold, and other metals fall off the bottom of the bath as a mud, and the pure copper is deposited on thin plates which have been previously black-leaded. The silver, gold, fragments of copper, and other metals in the mud are screened to eliminate coarse pieces of copper, and the finer slimes treated for their gold and silver contents.
An analysis of this slime was kindly supplied by Mr. Blakemore, general manager to the company:
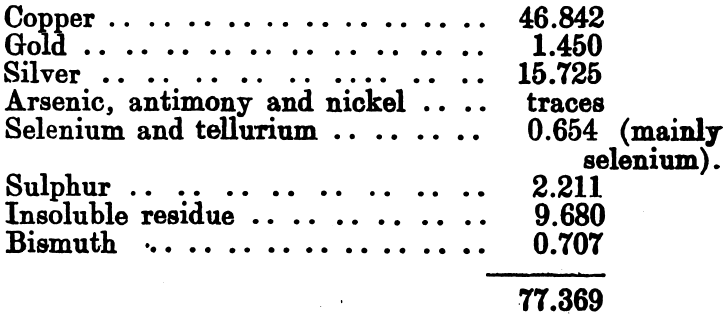
The remaining 23 per cent, consisted of graphite, grease, and other admixed impurities.
Another analysis supplied by Mr. Radcliff, from the Moonta electrolytic slimes, is as follows:
Gold………………………………………………… 0.585
Silver……………………………………………….. 7.865
Copper……………………………………………… 32.11
CuSO4………………………………………………. 11.33
PbSO4………………………………………………. 23.32
CaSO4………………………………………………. 0.535
S ……………………………………………………….3.940
Fe………………………………………………………..0.98
M……………………………………………………….0.205
Zn………………………………………………………0.140
As…………………………………………………………1.47
Sb2S3……………………………………………………7.10
AS2O3…………………………………………………..1.99
Bi……………………………………………………….0.075
Te……………………………………………………….0.123
Cl…………………………………………………………0.26
Mg………………………………………………………..0.14
Insol……………………………………………………..1.18
H2O at 120deg……………………………………….1.74
Not determined…………………………………….5.95
The removal of the copper from the slimes is best done by adding sulphuric acid and air. Solution takes place in accordance with the following equation:
Cu + H2 SO4 + O = Cu SO4 + H2O
The acid used is usually 90 per cent, strength, but a much weaker acid will suffice. The slimes are introduced into a lead-lined vat, and the solution heated by means of a steam coil. Hot air is forced in through an injector and heating and agitation are kept up for about twelve hours. Nearly all the copper, and 66 per cent, of the arsenic, and all the bismuth and iron are dissolved. The author carried out experiments with 50 per cent, sulphuric acid, and found that the whole of the copper could be passed into solution if the heating and air supply were continued, and that usually the undissolved copper consisted of coarse fragments which were too coarse to dissolve within the time allowed.
The slimes were then allowed to settle, and the supernatant solution decanted. Should all the copper not have dissolved, silver sulphate obtained from a subsequent operation is added, the silver rapidly replaces the copper:
Cu + Ag2 SO4 = Cu SO4 + 2 Ag.
If too much silver sulphate were added, the addition of a small amount of raw slimes will suffice to precipitate the soluble silver. The vat is filled to the original level with water, and the solution again boiled by means of the steam coil. The slimes are washed several times by decantation, and finally filtered. They are then dried and smelted with sand and sodium carbonate in a reverberatory furnace. The resulting dore bullion is then parted with sulphuric acid.
Experiments carried on with nitre cake on this class of material gave promising results. Since there is no silica, calcium sulphate, or other insoluble substances which tend to form the pink powder before referred to, the gold runs together well, even when in small quantities, and after washing out the soluble salts the insoluble compounds, such as antimony and bismuth basic salts, can be mechanically washed away. When the amount of silver is comparatively small the nitre cake process might be worked with advantage, but when the amount of gold is very small the direct fusion in a reverberatory furnace, followed by electrolytic or sulphuric acid parting, affords a simple method of dealing with this material.
How to Separate Gold from Platinum and Iridium
The bullion containing gold, platinum, and iridium, is dissolved in aqua regia, and the filtrate evaporated to dryness in a water bath. Nitric acid is expelled by evaporating repeatedly after the addition of hydrochloric acid. The chlorides are dissolved in a minimum amount of water. To the concentrated solution, chloride of ammonium is added until a precipitate ceases to form. It is allowed to stand for 24 hours at 80deg. C. The platinum precipitate is washed with a solution made by taking a saturated solution of ammonium chloride and diluting it with one-third of its volume of water. Afterwards it is washed with hydrochloric acid. The filtrate is evaporated until the ammonium chloride crystals start to separate out. A violet crystalline salt contains any iridium present; these are filtered and washed with ammonium chloride solution. The contents of each filter is ignited. To separate any platinum from the sponge after ignition, the latter is treated with aqua regia diluted with five times its volume of water, and heated to 40deg C. By repeating the operation with dilute aqua regia until the solution ceases to be colored, the platinum is removed and the iridium left behind. The gold may be thrown down with ferrous sulphate.
Separation by Electrolysis
The separation of these metals may be effected by electrolysis, in which a dilute solution of auric chloride is used as an electrolyte. A low current density is used to deposit the gold in an adherent form. The metals of the iridium group separate as a grey black slime. The method of separating osmiridium from gold by means of the higher density of the latter alloy has already been described. Platinum may also be separated from gold when the metals are finely divided by means of sodium bisulphate or nitre. Usually the method is made use of for treating platiniferous gold after it has been parted from silver by means of sulphuric acid. After the silver has been removed the residual gold is heated in an iron crucible with twice its weight of sodium bisulphate for from two to three hours. The molten mass is poured on to an iron plate, and after cooling is washed in porcelain vessels with hot water. The residue is dried and slowly heated with nitre to remove the last trace of platinum. The heating with nitre is continued for five or six hours; after this it is kept in a molten state for about as long. There is no doubt this prolonged heating with nitre might be shortened by the use of sodium peroxide. The gold after this treatment is free from platinum, and will assay 998 fine.
The platinum is recovered by reduction with litharge. The lead is cupelled and the platinum remaining dissolved in aqua regia, and the platinum precipitated as before described with ammonium chloride.
Purification of Bars of Base Bullion by Means of Sulphur and Soda
Since the foregoing was written, the author has carried out many experiments on the elimination of base metals from gold-silver bullion by means of sulphur. Bars of base bullions are purified most easily by adding carbonate of sodium, and afterwards allowing sulphur to flow or drip into the pot. The sulphur may be always kept in excess by this means, the sodium polysulphide formed acting as a carrier to the base metals. In this case, gold is passed into the sulphide matte, which floats above the molten metals, and it is somewhat remarkable that although the latter may still carry notable quantities of copper and silver, these do not serve to reduce the gold present as a sulphide in the matte.
The gold present in the matte is easily extracted by melting the slag or matte separately and adding sufficient iron. The amount necessary can be told by inspection of the reduced gold from time to time.
