Table of Contents
- Raw Materials
- Equipment
- Experimental Procedure
- Operation of Test
- Tests Under Atmospheric Pressure
- Experimental Results
- MgCl2 Brine
- Effect of Temperature at Atmospheric Pressure
- Effect of Time at Temperature at Atmospheric Pressure
- Effect of Temperature at Reduced Pressures
- Effect of Time at Temperature at Reduced Pressures
- Effect of Pyridine Hydrochloride Excess
- Spray-Dried MgCl2
- Determining Composition of Spray-Dried MgCl2
- Investigating Effects of Variables
- Effect of Pyridine Hydrochloride Excess
- Effect of Temperature
- Effect of Time at Temperature
- Calculating Distribution of Water in Spray-Dried MgCl2
- Summary and Conclusions
The United States has almost limitless supplies of magnesium chloride available for the production of magnesium metal in the form of hydrated salts or brines from seawater, wells, and the Great Salt Lake. Anhydrous magnesium chloride has certain advantages for metal production but cannot be made from the hydrate or brines by simple drying. In keeping with its goal of maximizing minerals and metals recovery from domestic resources in order to assure an adequate supply of minerals to meet national economic and strategic needs, the Federal Bureau of Mines investigated a novel technique for making anhydrous magnesium chloride from hydrated salts or brines.
More than 95 percent of the current U.S. magnesium metal output is produced by two electrolytic processes. These processes are often designated as the “Dow” process and the “I. G. Farbenindustrie” (IGF) process. A primary difference between the two processes is that the Dow process uses partially hydrated magnesium chloride as cell feed, whereas the IGF process requires an anhydrous cell feed. The use of partially hydrated cell feed is an advantage for the Dow process, but the presence of water increases the carbon anode consumption and also reduces current efficiencies. The offgas from the IGF cell is primarily chlorine, which can be marketed as a byproduct, while the Dow cell offgas is a mixture of Cl2, HCl, CO2, air, and water vapor. Much of the Dow cell offgas is recycled to the process and not recovered as a byproduct.
A significant portion of current magnesium production expansion in the United States uses adaptions of the IGF process for the production of magnesium metal from magnesium-rich brines. These brines are obtained from under-ground solution mining operations or are produced by solar evaporation of surface brines such as those found in the Great Salt Lake of Utah.
Dehydration of magnesium chloride is not easily accomplished. Magnesium chloride is very water soluble and crystallizes from solution as the hexa-hydrate, MgCl2·6H2O. This salt cannot be dehydrated by simple heating because magnesium oxychlorides and magnesium oxides are formed with the evolution of hydrogen chloride. Spray-drying of the brine produces a magnesium chloride product containing minor amounts of magnesium oxide and water.
A wide diversity of methods have been proposed for producing anhydrous magnesium chloride. Methods involving gas-solid reactions are chlorination of magnesium oxide or partially dehydrated magnesium chloride and thermal decomposition of the hexahydrate in a stream of hydrogen chloride gas. Disadvantages of these methods are the presence of large quantities of corrosive gases, the need to maintain essentially anhydrous conditions, and the high electrical energy input required to heat the charge to reaction temperature.
Another approach to dehydration starts with ammonium carnallite (NH4Cl·MgCl2·6H2O), which is heated in two stages to drive off water and then to sublime NH4Cl. Collection of NH4Cl for recycle requires condensation, a difficult operation to carry out on a large scale.
Other patents show heating hydrous forms of magnesium chloride with an alcohol ; with N,N dialkylamides ; and with various organic compounds containing sulfur, oxygen, phosphorus, or nitrogen. However, none of these methods show the formation of magnesium chloride from any magnesium oxides that may be present. Although this listing of dehydration methods is not complete, it does describe the wide range of approaches to dehydration and illustrates the difficulties of the problem.
In 1971, the use of organic amine hydrochlorides for dehydrating magnesium chloride brines and hydrates was proposed. Pioneering tests showed that organic amine hydrochlorides form stable double salts from which water of hydration can be driven off without decomposition of the salt, and organic amine hydrochlorides possess acid characteristics at high temperature which enable them to chlorinate magnesium oxides. In addition to these chemical properties, which are similar to those of ammonium chloride, some organic amine hydrochlorides have relatively low melting and boiling points that would make for a manageable dehydration process. A patent was issued to Dolezal for this method.
The double-salt dehydration process, as patented, can be divided into four steps, some of which take place simultaneously. Reactions with both brine and the solid hydrate are illustrated by the following reactions, in which the organic amine hydrochloride is shown as RN-HCl.
- Magnesium chloride hydrates and brines, which may contain magnesium oxide as impurity, are contacted with an excess of organic amine hydrochloride. A complex of the organic amine hydrochloride and magnesium chloride hydrate is formed. If a brine is used, the complex dissolves in the brine.
MgCl2 Brine + RN·HCl = RN·HCl·MgCl2·6H2O + HgO
MgCl2 Hydrate + RN·HCl = RN·HCl·MgCl2·nH2O - The complex is heated to drive off water, leaving an anhydrous equi-molar addition compound, a double salt.
RN·HCl·MgCl2·6H2O = RN·HCl·MgCl2 + 6H2O (g)
RN·HCl·MgCl2·nH2O = RN·HCl·MgCl2 + nH2O (g) - The magnesium chloride-organic amine hydrochloride compound is decomposed into anhydrous magnesium chloride and organic amine hydrochloride. Any magnesium oxide present is converted to magnesium chloride with formation of the amine.
RN·HCl·MgCl2 = RN·HCl (g) + MgCl2
MgO + 2RN·HCl = 2RN (g) + MgCl2 + H2O (g) - The volatilized amine hydrochloride and any free amine that is formed are collected for recycle. Hydrochloric acid is added as required to convert the amine to the hydrochloride.
RN + HCl = RN·HCl
The Bureau of Mines investigated the application of double-salt decomposition to the dehydration of magnesium chloride brines and of spray-dried magnesium chloride. This report describes the raw materials, the laboratory equipment used, and the effects of temperature, reduced pressure, time, and excess of organic amine hydrochloride on dehydration. Early tests were run with both aniline and pyridine hydrochloride. The final tests reported here were run with pyridine hydrochloride, which has the lower melting and boiling points of these two amine salts. The criteria used to evaluate the effects of variables were (1) oxygen content of the product to show the extent of dehydration of the magnesium chloride, and (2) organic amine hydrochloride content in the anhydrous product to show that the organic amine hydrochloride was volatilized for recovery.
Raw Materials
Two feed materials were used for dehydration tests: (1) Synthetic solutions of MgCl2 brine and (2) spray-dried MgCl2 obtained from an industrial operation. In addition, five samples of anhydrous MgCl2 were obtained from industrial sources to provide a basis for comparison between industrial cell-feed materials and the anhydrous MgCl2 products of our dehydration tests.
35 Percent MgCl2 Brine
Synthetic solutions of 35 percent MgCl2 were prepared to simulate brine that is the end product of evaporating the waters of Great Salt Lake. Solar evaporation is used to crystallize NaCl, KCl, Na2SO4, and double salts of potassium, magnesium, chloride, and sulfate. After these salts are removed, a concentrated brine remains that is 35 percent MgCl2 by weight. For the experimental work described in this report, the synthetic test solutions were prepared by dissolving MgCl2·6H2O in water.
Spray-Dried MgCl2
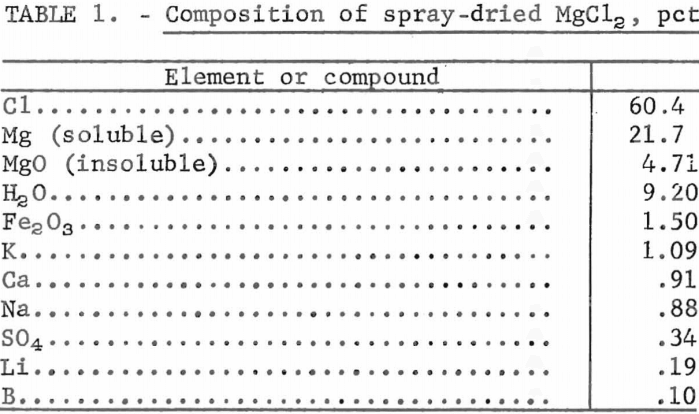
In the recovery of magnesium from brines by the IGF process, brines are spray-dried at 600° C to obtain an intermediate product of magnesium chloride which contains about 5 percent MgO, 5 to 10 percent water, and the minor impurities that are present in the concentrated brines. Most of the oxygen and water content must be removed by rechlorination before this MgCl2 can be used in IGF electrolytic cells. Composition of spray-dried MgCl2 is shown in table 1.
Anhydrous MgCl2
The anhydrous MgCl2 samples used for comparison were from two sources:
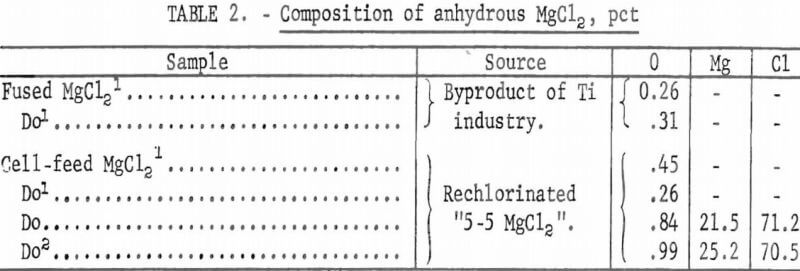
(1) Fused MgCl2, which is a byproduct of production of titanium by the Kroll process, and (2) MgCl2 cell-feed material prepared by rechlorinating the “5-5 MgCl2.” These samples were analyzed for oxygen and for minor impurities. Composition of anhydrous MgCl2 is shown in table 2.
Organic Amine Hydrochlorides
Of the organic amine hydrochlorides listed in the proposed dehydration process patent, aniline hydrochloride-melting point 198° C; boiling point 245° C and pyridine hydrochloride melting point 145° C; boiling point 219° C were used in this investigation because of their low melting and boiling points and because they are readily available. These compounds were obtained from chemical supply companies and were used without purification. Pyridine hydrochloride was completely soluble in water; aniline hydrochloride contained a very small amount of insoluble impurities, mainly iron and copper.
Equipment
Preliminary tests indicated that the equipment should be designed in such a way that (1) the reaction flask could be heated uniformly and close temperature control maintained, (2) the anhydrous MgCl2 product could be sampled without exposure to the air, and (3) the dehydration-decomposition reaction could be conducted under reduced pressure. The apparatus constructed to conform to these requirements was used for all of the tests described in this report. The equipment consisted of (1) a round reaction flask, (2) a heated crossover, and
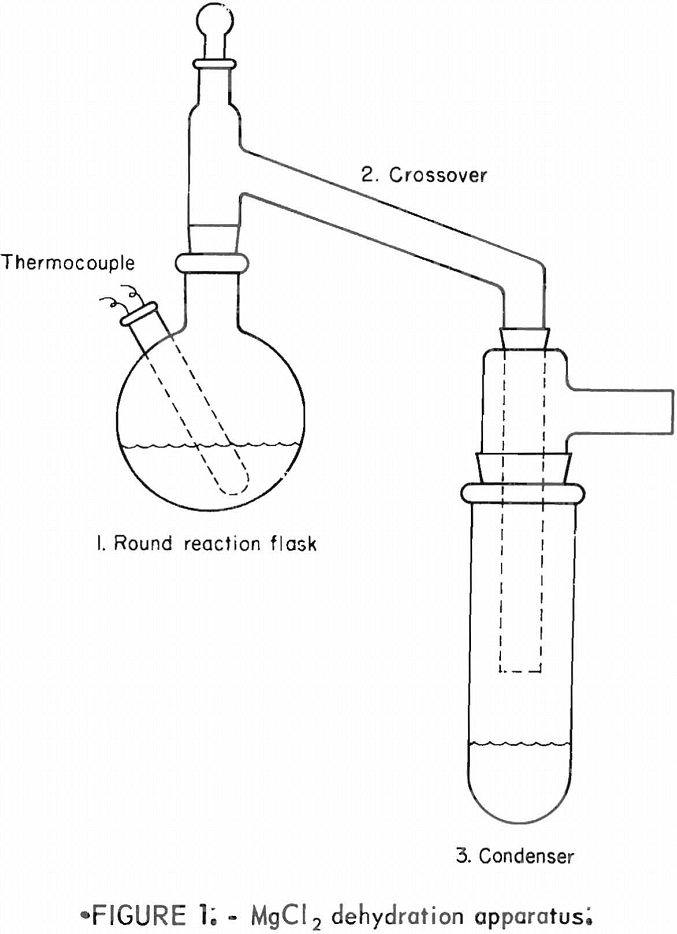
(3) an ice-cooled condenser (fig. 1). These three components were of Pyrex glass with standard taper joints and fittings. The flask was heated in a fluidized sand bath. The desired temperature within the reaction chamber was maintained by controlling the power input to the sand bath. The crossover was heated to over 100° C by a heating tape. The condenser was set in an ice- methanol bath.
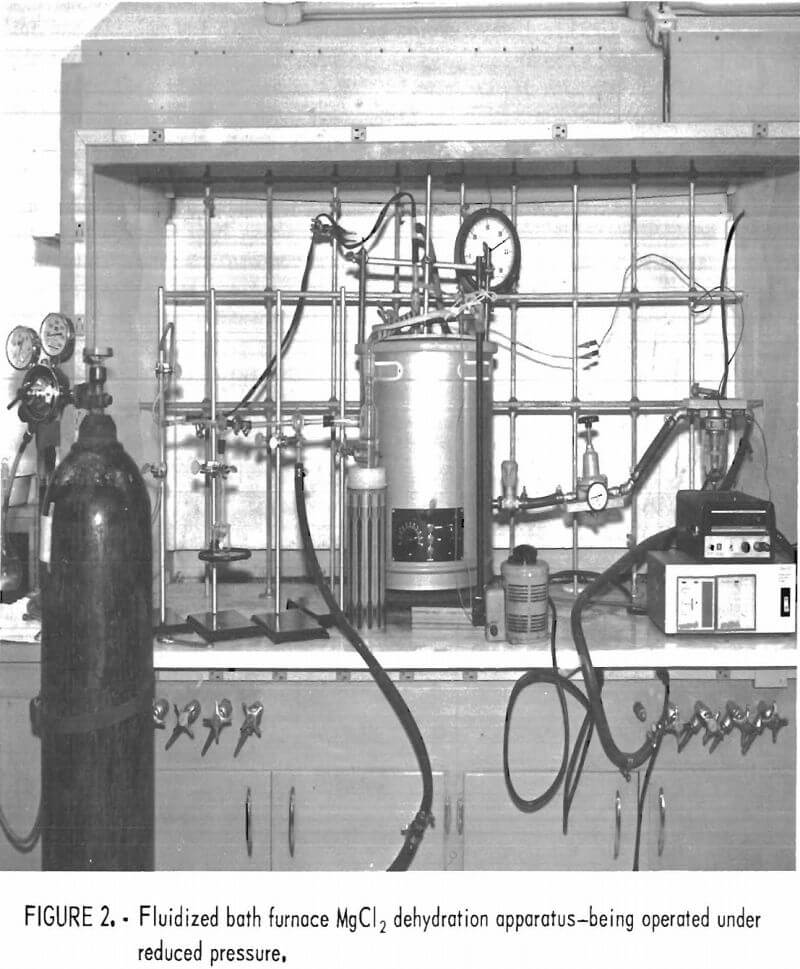
Figure 2 shows the apparatus being operated under reduced pressure. The outlet from the cold trap is connected to a three-way stopcock, which can be turned to connect to atmospheric pressure or to the vacuum pump. To permit backfilling the reaction flask with nitrogen, the top of the crossover directly above the vacuum flask is connected by rubber tubing to the nitrogen tank through a standard taper joint. The vacuum gage connection is also positioned at the top of the crossover.
Experimental Procedure
Standardized experimental procedures were followed so that results obtained for a series of tests could be compared directly.
Preparation of Feed Mixtures
35 Percent MgCl2 Brine
Synthetic solutions of MgCl2 brine were prepared by dissolving 44.85 grams of MgCl2·6H2O (found by analysis to contain 49.2 percent MgCl2) in 18.15 grams of water. Weighed amounts of pyridine hydrochloride were dissolved in this solution. A 10-milliliter aliquot was taken and diluted to 500 milliliters for feed analysis. The remaining solution was poured into the reaction flask.
Spray-Dried MgCl2
Spray-dried MgCl2 was stored in a sealed container. To prepare feed for each test, 25 grams of spray-dried MgCl2 was weighed and dry-mixed with a weighed amount of pyridine hydrochloride. The dry mixture was poured into the reaction flask. To obtain a feed material analysis with each test, 12.5 grams of spray-dried MgCl2 was dissolved in 500 milliliters of water. The insoluble portion was removed by filtration, dried and weighed. The soluble and insoluble portions were analyzed for chlorine and magnesium. In addition, samples were taken from the container under an inert atmosphere on the day of the test and sent for oxygen analysis along with samples of the anhydrous MgCl2 product of that test.
Operation of Test
The operating procedures are described in detail because careful attention proved to be necessary for obtaining reproducible data.
Tests Under Atmospheric Pressure
The thermocouple was positioned in the thermocouple well of the reaction flask and the flask was positioned in the furnace. The prepared feed mixture was poured into the flask, and the crossover, cold trap, and connection to nitrogen were put into place. The crossover was wrapped with a heating tape controlled by a variable transformer, and the cold trap was surrounded with an ice-methanol mixture. A slight flow of the nitrogen was turned on to the connecting bubbler.
The sand bath was fluidized with air, the furnace was turned on, and controller was set for the test temperature. The crossover heating tape was set to maintain a temperature between 100° C and 125° C. The temperature in the reaction flask was monitored by the thermocouple in the sidewell. When the test temperature was reached, usually after 1 to 2 hours, this temperature was maintained by minor manual adjustments in the controller setting for the length of time set for the test.
At the end of test time, the furnace controller, fluidizing air, and heating tape controller were turned off. The crossover was removed from the reaction flask and the flask was immediately sealed with a standard taper stopper. This step must be carried out quickly and smoothly to insure that the hot anhydrous product is exposed only momentarily to the atmosphere.
Tests Under Reduced Pressure
Two levels of vacuum were used for tests under reduced pressure. An intermediate level was obtained from the building vacuum system. The pressure in this system fluctuated between minus 12 and minus 18 inches mercury. A higher level of vacuum was obtained from a small, mechanical vacuum pump in the laboratory which produced a consistent pressure of minus 23.5 inches mercury. In this report, the two levels of vacuum will be referred to as “minus 12 to minus 18 inches mercury” and “minus 23.5 inches mercury.”
The operating steps for tests under reduced pressure were the same as for tests under atmospheric pressure to the point at which the test temperature was reached. At that time, the three-way stopcock was turned to the vacuum source. When vacuum was applied, the temperature in the reaction flask dropped as much as 30° C, owing to heat absorbed by vaporization of water and pyridine hydrochloride. The temperature returned to test temperature within 15 to 30 minutes. The test time was measured from the first time temperature was reached.
Handling of Products
The two products recovered from MgCl2 dehydration tests were anhydrous MgCl2 remaining in the reaction flask, and the pyridine hydrochloride and water condensed in the crossover and cold trap.
Anhydrous MgCl2 Product
When the reaction flask had cooled, it was weighed and taken to a nitrogen-filled dry box so that samples for oxygen determination could be taken without exposing the product to air. The product, which was a friable solid, was ground. Special sample vials used in the neutron activation equipment for oxygen analysis had been provided by the Reno Metallurgy Research Center. Two of these vials were filled, sealed, and mailed to Reno on the day of the test.
The remaining MgCl2 was returned to the flask. The flask was reweighed and the contents dumped into about 800 milliliters of water. The flask was rinsed and the solution was diluted to 1 liter. This solution was analyzed for pyridine hydrochloride, magnesium, and chloride. Residues from tests with spray-dried MgCl2 were also analyzed for minor impurities.
Collected Pyridine Hydrochloride
The collected pyridine hydrochloride solid was washed from the crossover and cold trap and combined with the liquid from the cold trap. The solution was diluted to 1 liter. This solution was analyzed for pyridine hydrochloride, magnesium, and chloride.
Analysis of Products
Analyses for magnesium and chloride were by conventional methods, but the oxygen and pyridine hydrochloride analyses required specialized equipment and techniques. Oxygen analyses were done at the Reno Metallurgy Research Center by fast neutron activation analysis. An analytical method for pyridine hydrochloride using high-pressure liquid chromatography and ultraviolet detection was developed at the Salt Lake City Metallurgy Research Center.
Experimental Results
The goal set for maximum oxygen content of the anhydrous magnesium chloride produced in the dehydration tests was 0.8 percent or less because available information indicated that magnesium chloride used as cell-feed contained about this level of oxygen (table 2). The goal set for pyridine hydrochloride content in the anhydrous product was less than 2 percent.
MgCl2 Brine
The four variables investigated with magnesium chloride brine as feed material were (1) temperature; (2) pressure, atmospheric or reduced pressure; (3) time at temperature; (4) excess of pyridine hydrochloride over the stoichiometric requirement of 1 mole pyridine hydrochloride for 1 mole of magnesium chloride.
Effect of Temperature at Atmospheric Pressure
Previous tests showed that temperature had a greater effect than time on removing pyridine hydrochloride from the anhydrous magnesium chloride product. Moreover, the results indicated that the temperatures required were well above 219° C, the boiling point of pyridine hydrochloride. The need for higher temperatures suggested that additional heat was required to dissociate the double salt of magnesium chloride and pyridine hydrochloride and, thereby, free pyridine hydrochloride for removal by vaporization.
A series of tests was run at temperatures increasing from 375° to 475° C at atmospheric pressure. Results are shown in table 3. Because reporting oxygen content in the total product would be misleading, the results are given as oxygen, percent, in the magnesium chloride portion of the product. For example, in the product from the test run at 375° C, the oxygen in the total product was 0.36 percent but, because the product was 28 percent pyridine hydrochloride, oxygen in the magnesium chloride portion was 0.50 percent.
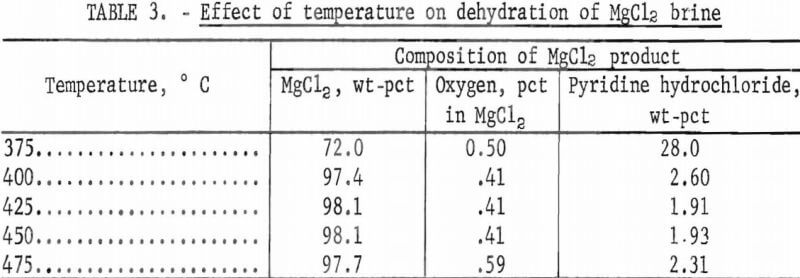
This table shows that anhydrous magnesium chloride was formed at all temperatures; the oxygen contents in the magnesium chloride portions of the residues were below 0.8 percent. Removal of pyridine hydrochloride was very sensitive to temperature; the critical temperature was between 375° and 400° C. However, pyridine hydrochloride removal was not increased significantly when temperatures were increased from 400° to 475° C.
Effect of Time at Temperature at Atmospheric Pressure
A series of tests was run at 400° C for one-half, 1, and 2 hours to determine the effect of time at temperature on removal of pyridine hydrochloride from the anhydrous product. These tests were run at atmospheric pressure with 25 percent excess pyridine hydrochloride in the brine. The results (table 4) showed that prolonging the time that the magnesium chloride product was held at temperature did not increase the amount of pyridine hydrochloride removed from the product.
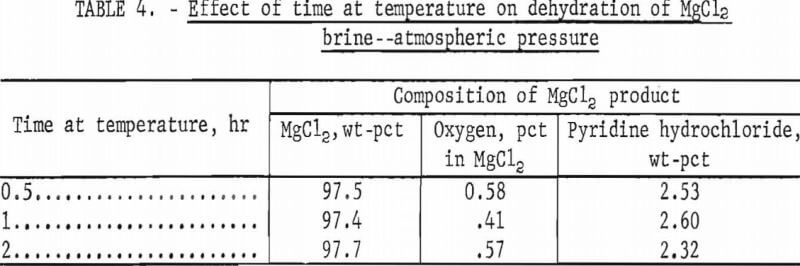
Conditions: Atmospheric pressure, 400° C, 25 pct excess pyridine hydrochloride.
Effect of Temperature at Reduced Pressures
Anhydrous magnesium chloride products were obtained from the tests run at atmospheric pressure regardless of the temperatures and times of the tests. The purpose of tests run under reduced pressure was to determine whether applying a vacuum to the reaction flask would facilitate vaporization of pyridine hydrochloride at temperatures lower than 400° C.
Two series of tests were run-the first at minus 12 to minus 18 inches mercury, and the second at minus 23.5 inches mercury. In all the tests, the reaction flask was heated to test temperature under atmospheric pressure, and then the three-way stopcock following the cold trap was switched to the source of the vacuum. For each series of tests, the first test was run at 400° C, and the following tests were run at decreasing temperatures until the minimum temperature for effective pyridine hydrochloride removal was determined. Results are given in table 5.
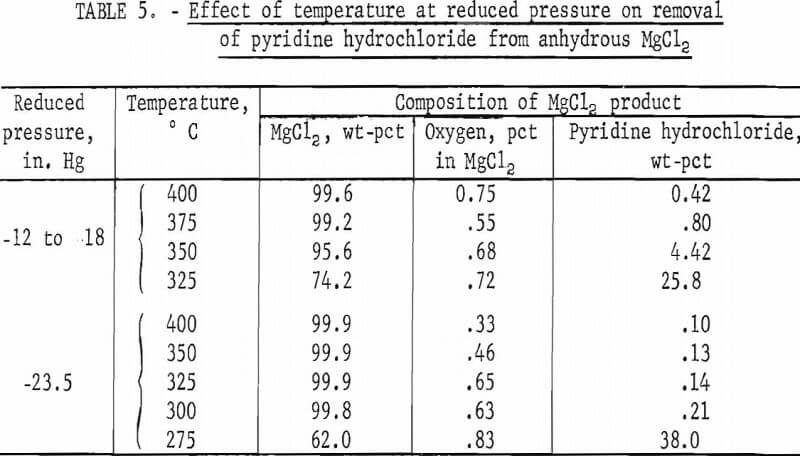
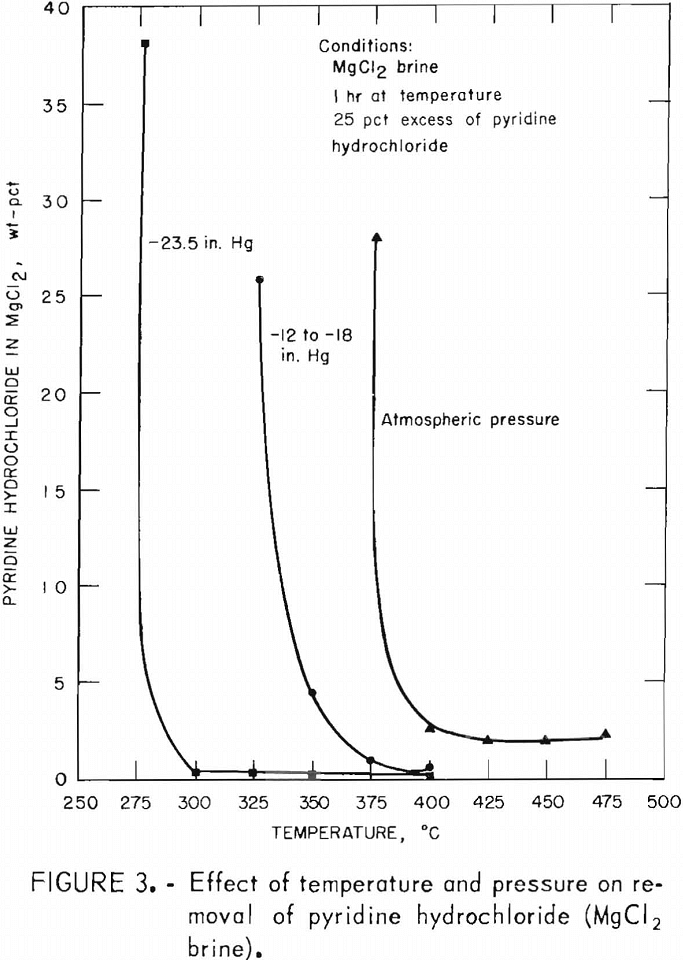
Results of the three series of dehydration tests determining effects of tem-perature and of atmospheric and two levels of reduced temperature on pyridine hydrochloride removal are summarized in figure 3.
Table 5 and figure 3 show clearly that under reduced pressure pyridine hydrochloride was removed from the anhydrous magnesium chloride products at temperatures much lower than those required under atmospheric pressure. The minimum temperatures required for removal of pyridine hydrochloride for each of the pressures were about 75° C apart:
450° C at atmospheric pressure
375° C at minus 12 to minus 18 inches mercury
300° C at minus 23.5 inches mercury
In addition, the use of reduced pressure resulted in magnesium chloride products containing as low as 0.1 percent pyridine hydrochloride in contrast to 1.9 percent found in the best products from tests under atmospheric pressure. Again, there is a sharp distinction between the temperatures at which pyridine hydrochloride is removed and the temperature, 25° lower, at which over 25 percent of the anhydrous product is pyridine hydrochloride.
Effect of Time at Temperature at Reduced Pressures
Two series of tests were run with minus 12 to minus 18 inches mercury to determine the effect of time on removal of pyridine hydrochloride. The first series was run at 375°, the minimum effective temperature determined in 1-hour tests. The second series was run at 350° C, the temperature that in 1-hour tests appeared to be very near the critical temperature for pyridine hydrochloride removal. Results are shown in table 6.
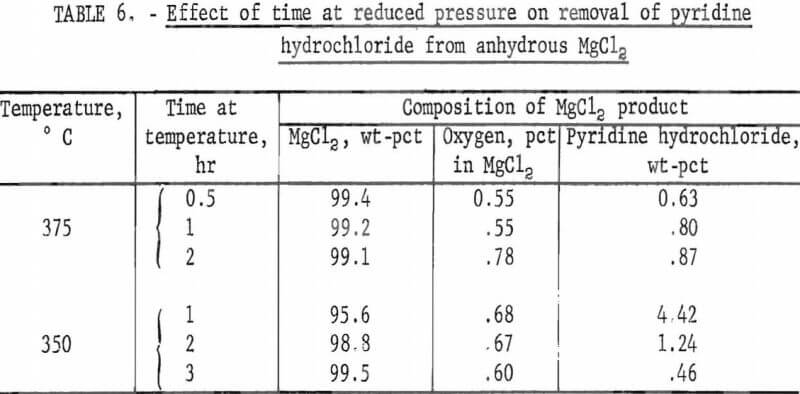
Conditions: Pressure -12 to -18 in. Hg, 25 pct excess pyridine hydrochloride
This table shows that prolonged heating does not increase removal of pyridine hydrochloride when the test temperature is above the minimum required; at 375° C, one-half hour was as effective as 1 hour. But at 350° C, longer times at temperature under vacuum did result in increased pyridine hydrochloride removal. Apparently, a combination of time and temperature could be defined that would be the most efficient for removing residual pyridine hydrochloride.
Two time tests run with minus 23.5 inches mercury showed that at 300° C the pyridine hydrochloride content in the anhydrous product was 0.75 percent after one-half hour and 0.21 percent after 1 hour. One-half hour was the time required for the temperature in the reaction flask to return to 300° after vacuum was applied. Both of these amounts of pyridine hydrochloride are well below the goal of 2 percent that was set at the start of the investigations.
Effect of Pyridine Hydrochloride Excess
The effect of pyridine hydrochloride excess on dehydrating MgCl2 brine was studied in two limited series of tests at atmospheric and at reduced pressure. The amount of pyridine hydrochloride excess affects the oxygen content in the anhydrous MgCl2 product.
The reaction of MgCl2·6H2O and organic amine hydrochloride had been studied by thermogravimetric analysis before investigations were begun in laboratory equipment. In thermogravimetric analysis, the weight of the sample is continuously recorded while the sample is heated at a steady rate. One object of this study was to determine whether 1 mole of organic amine hydrocloride is the minimum required for dehydration of each mole of MgCl2·6H2O or whether a lower mole ratio would be effective as long as some organic amine hydrochloride is present while MgCl2·6H2O is being dehydrated. For the thermogravimetric study, the two compounds were mixed in mole ratios that ranged from 1.6:1 to 0.1:1 for organic amine hydrochloride: MgCl2·6H2O. A sample of MgCl2·6H2O only was run for comparison. The weight recordings showed that weight loss corresponded to formation of MgCl2, MgOHCl, or a mixture of the two compounds above 250° C. The results can be grouped by samples:
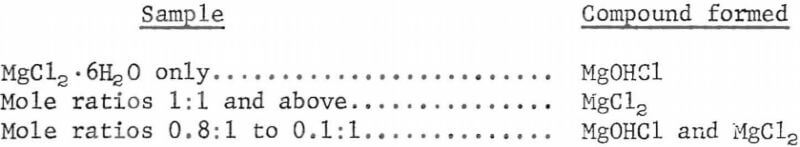
The mixtures of MgOHCl and MgCl2 roughly compared to the deficiency of organic amine hydrochloride; that is, the less organic amine hydrochloride in the mixture the more MgOHCl was formed. These tests proved that at least 1 mole of organic amine hydrochloride must be provided for 1 mole of magnesium chloride to obtain anhydrous magnesium chloride by double-salt decomposition.
Therefore, initial tests in the fluidized bath furnace were run with 25 percent excess of pyridine hydrochloride. After the effects of temperature, pressure, and time had been investigated, two tests were run with lower amounts of pyridine hydrochloride mixed with magnesium chloride brine. These tests were run at 375° C for 1 hour at temperature- The results are shown in table 7.
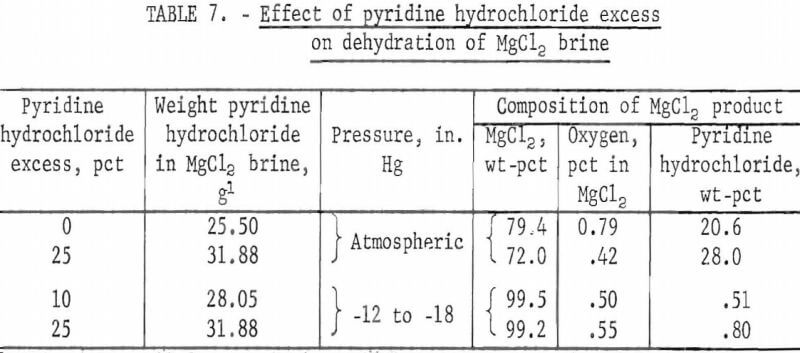
Results of these limited tests suggest that the minimum excess of pyridine hydrochloride required to dehydrate magnesium chloride brine lies between zero percent stoichiometric requirement and 10 percent. Further tests would be needed to determine the excess precisely.
Spray-Dried MgCl2
Whereas dehydration of magnesium chloride brine is straightforward, production of anhydrous magnesium chloride from spray-dried MgCl2 by double-salt decomposition entails three possible reactions:
- Chlorination of magnesium oxide
MgO + 2 C5H5N·HCl (pyridine hydrochloride) = MgCl2 + 2 C5H5N + H2O, - Chlorination of magnesium hydroxychloride (MgOHCl)
MgOHCl + C5H5N·HCl = MgCl2 + C5H5N + H2O, and - Dehydration of hydrated magnesium chloride
MgCl2·nH2O + C5H5N·HCl = MgCl2 + C5H5N·HCl + nH2O.
Before the stoichiometric requirements for these reactions can be calculated, the distribution of oxygen in spray-dried MgCl2 as MgO and MgOHCl or MgCl2·nH2O must be determined.
Determining Composition of Spray-Dried MgCl2
Spray-dried MgCl2 was analyzed in conjunction with each dehydration test. Therefore, the composition shown in table 1 is the averaged result of 18 analyses. To determine oxygen content two samples were taken under inert atmosphere and sent for neutron activation analyses along with the anhydrous products obtained from each test. To determine magnesium oxide, soluble magnesium chloride, and minor impurities, 12.5 grams of spray-dried MgCl2 was dissolved in 500 milliliters of water. This solution was filtered; the insoluble portion when dried weighed about 1.33 grams and contained 28.4 percent magnesium and 10.5 percent iron. The soluble portion was 21.7 percent magnesium and 60.4 percent chloride, giving the mole ratio Cl:Mg = 1.9:1. Average oxygen analysis was 10.5 percent. Contrary to expectations, spray-dried MgCl2 was very stable; the oxygen content did not go up as the container was reopened during the 4-month test period but varied between 11.6 and 9.95 percent.
The water content of the spray-dried product shown in table 1 was calculated by subtracting oxygen in magnesium oxide from total oxygen determined by neutron activation analyses. Although this oxygen remainder is reported as water, it was not determined whether the water is present as combined with magnesium chloride as hydroxyl ion (MgOHCl), as combined with magnesium chloride as a hydrate (MgCl2·nH2O), or as adsorbed water. In any case, reactions 2 and 3 show that 1 mole of pyridine hydrochloride is required for each mole of MgOHCl or MgCl2·nH2O. If water is present as adsorbed water, it will be vaporized by heat and no pyridine hydrochloride will be required.
Investigating Effects of Variables
Because spray-dried MgCl2 contained oxygen-bearing impurities, the goal for oxygen in anhydrous products from these tests was set at 1.26 percent.
Spray-dried MgCl2 feed material contained 0.34 percent sulfate (SO4) and 0.10 percent boron which is present as borate (B2O3). These impurities are not chlorinated, but remain at the same levels in the anhydrous products. This fact was confirmed by analyses of the anhydrous products for impurities. The net weight loss from spray-dried MgCl2 to anhydrous magnesium chloride is 2.8 per-cent; that is, the loss of 9.2 percent due to dehydration is offset by the gain of 6.4 percent due to chlorination of magnesium oxide-replacement of oxygen with chlorine. Consequently, the anhydrous products contained 0.35 percent sulfate and 0.33 percent borate. These two impurities will account for 0.46 percent oxygen in the product. Addition of this amount of oxygen to the 0.8 percent goal set for magnesium chloride brine tests gave a goal of 1.26 percent oxygen for tests with spray-dried MgCl2.
The goal for residual pyridine hydrochloride content in the anhydrous product was 2 percent-the same as that set for magnesium chloride brine tests- because the nature of the feed material should not affect the double-salt decomposition reaction.
All tests were conducted at atmospheric pressure.
Effect of Pyridine Hydrochloride Excess
The excess of pyridine hydrochloride mixed with spray-dried MgCl2 was studied first because this variable affects the oxygen level in the anhydrous product. The stoichiometric requirements for chlorination and dehydration of spray-dried MgCl2 are 2 moles of pyridine hydrochloride for 1 mole of magnesium oxide and 1 mole of pyridine hydrochloride for 1 mole of water. The amounts of pyridine hydrochloride used were calculated to be stoichiometric and 25, 50, and 100 percent excess based on the first analysis received (5 percent magnesium oxide and 8.5 percent water). Although these two values were found to vary as more analyses were run, the weights of pyridine hydrochloride used throughout the series of tests were held constant so that results would be comparable. Therefore, the amounts of pyridine hydrochloride tested were actually 3 percent less than stoichiometric, and 21, 43, and 94 percent excess based on the composited analyses of spray-dried MgCl2 (4.71 percent magnesium oxide and 9.2 percent water).
The effect of pyridine hydrochloride excess was investigated at 400° and 425° C. These tests were run for 1 hour at temperature. Results of these series are shown in table 8.
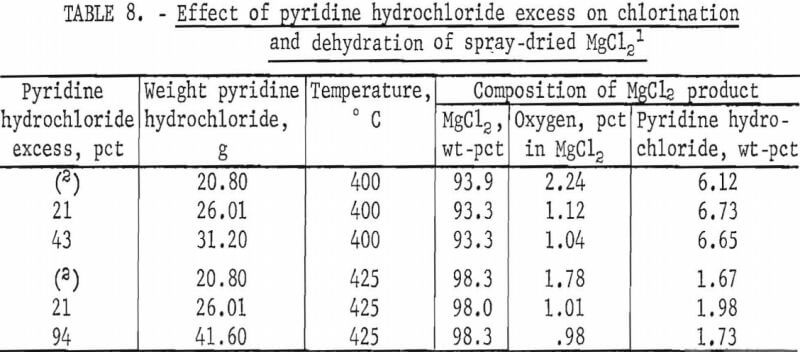
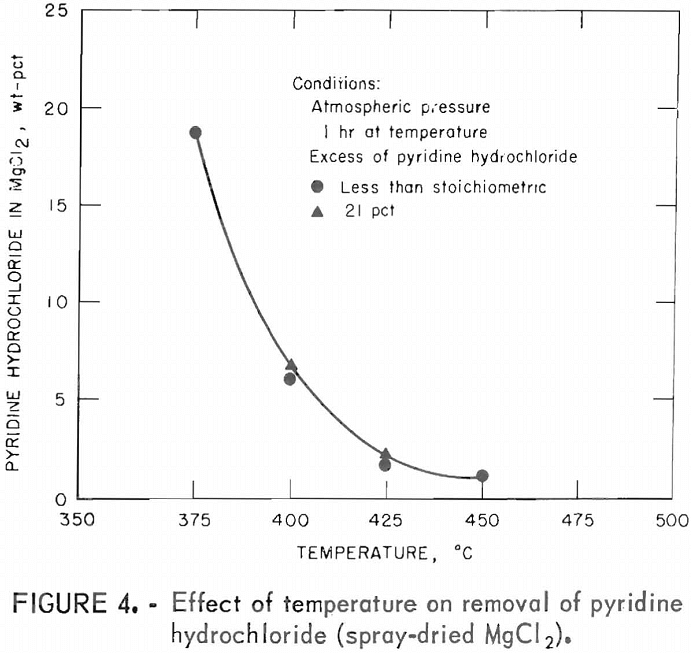
Table 8 shows that use of an excess of 20 percent pyridine hydrochloride produced anhydrous magnesium chloride containing 1.1 or less percent oxygen which meets the goal set for the tests. Use of larger amounts of pyridine hydrochloride did not reduce the oxygen levels significantly. Although the minimum was not precisely determined, certainly the stoichiometric requirement must be satisfied. This result confirms that the water content of spray-dried MgCl2 is combined as MgOHCl or MgCl2·nH2O and not simply present as adsorbed water which would have been driven off at a temperature much lower than 400° C.
Effect of Temperature
The effect of temperature on removal of pyridine hydrochloride was investigated with feed mixtures containing the amount of pyridine hydrochloride calculated to be the stoichiometric requirement. When further analyses proved this amount of pyridine hydrochloride to be deficient, tests were repeated with 21 percent excess. All tests were run for 1 hour at temperature.
Results are given in table 9 and illustrated in figure 4.
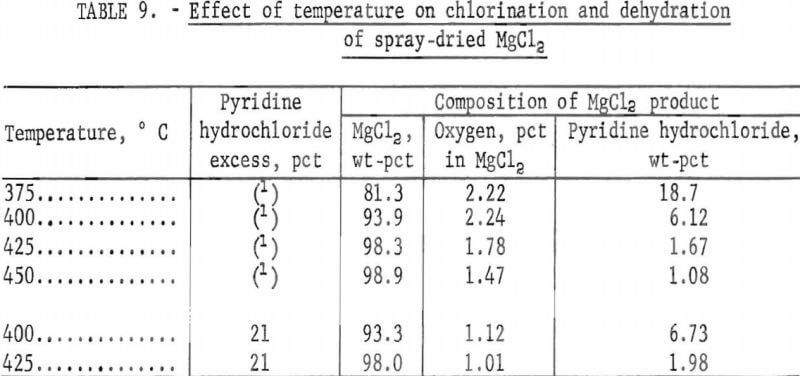
Table 9 and figure 4 show that removal of pyridine hydrochloride is dependent upon the test temperature and that the minimum temperature required to obtain an anhydrous product containing less than 2 percent pyridine hydrochloride was 425° C.
Effect of Time at Temperature
Results of tests with spray-dried MgCl2 showed that time at temperature had less effect than temperature on removal of pyridine hydrochloride as was the case with magnesium chloride brine as feed material. Comparable tests run at 425° C with 21 percent excess pyridine hydrochloride showed that the amount of pyridine hydrochloride remaining in the anhydrous product was 2.22 percent after 30 minutes and 1.98 percent after 1 hour. Doubling the time at temperature improved removal by only 0.24 percent.
Calculating Distribution of Water in Spray-Dried MgCl2
Although the distribution of water in spray-dried MgCl2 was not determined directly, the distribution could be calculated indirectly from the analysis of the products condensed in the crossover and collected in the cold trap. These two products combined contained the pyridine and pyridine hydrochloride evolved during the dehydration tests. The condensed solid was dissolved in water, added to the solution in the cold trap, and the final sample was analyzed for chloride ion and for pyridine. The excess of moles of pyridine over moles of chloride ion represents uncombined pyridine. The average weight of uncombined pyridine collected was 4.31 grams.
The following two of the reactions, which were postulated at the beginning of experimental work, result in evolution of pyridine: (1) Chlorination of MgO and (2) chlorination of MgOHCl. In chlorination of MgO, 2 moles of pyridine are evolved from each mole of MgO chlorinated. In chlorination of MgOHCl, 1 mole of pyridine is evolved for each mole of MgOHCl chlorinated. Based on 25 grams of spray-dried MgCl2 containing 4.71 percent of MgO and 9.2 percent water, the theoretical weights for pyridine evolved are 4.62 grams from chlorination of MgO and 10.10 grams from chlorination of MgOHCl. The amount of pyridine collected was 4.31 grams indicating that the pyridine evolved was from chlorination of MgO, not chlorination of MgOHCl.
Since the oxygen content of the product shows that the feed had been dehydrated, and since the amount of pyridine evolved shows that no MgOHCl is chlorinated, the water in spray-dried MgCl2 must be present as water of hydration- MgCl2·nH2O.
Summary and Conclusions
Laboratory-scale tests proved that anhydrous magnesium chloride was produced by decomposition of the double-salt of pyridine hydrochloride and magnesium chloride. Both 35 percent magnesium chloride brine and spray-dried magnesium chloride were used as feed materials.
Process variables investigated with magnesium chloride brine were temperature, time, excess of pyridine hydrochloride, and reduced pressure. Anhydrous magnesium chloride products containing less than 0.8 percent oxygen were obtained when an excess of 10 percent pyridine hydrochloride over the equi-molar requirement was present in the charge, at temperatures ranging from 300° to 475° C. Temperatures required to reduce pyridine hydrochloride to below 2 percent in the anhydrous product were 450° C at atmospheric pressure and 300° C at the reduced pressure of minus 23.5 inches of mercury. Increasing times at temperature did not significantly lower the amount of pyridine hydrochloride remaining in the anhydrous product.
Process variables investigated with spray-dried magnesium chloride were temperature, time, and excess of pyridine hydrochloride. Two moles of pyridine hydrochloride were required to chlorinate 1 mole of magnesium oxide and 1 mole of pyridine hydrochloride was required to dehydrate 1 mole of magnesium chloride hydrate. An anhydrous product containing less than 1.1 percent oxygen was obtained from spray-dried magnesium chloride when an excess of these stoichiometric requirements of pyridine hydrochloride was provided. At 425° C, the anhydrous product contained less than 2 percent pyridine hydrochloride. Results were only slightly improved for times at temperature greater than 0.5 hour.

