Table of Contents
Interest in the recovery of minerals from sea water has been stimulated in recent years by proposals for large-scale water desalination plants that would yield both fresh water and enriched brine. Extraction of selected mineral components from the brine or the sea water feed might help pay for the fresh water. Additionally, by processing sea water to remove scale-forming constituents before evaporation, heat transfer would be improved, and water production costs reduced.
A few years ago, we surveyed the prospects of minerals recovery from sea water and desalination plant bitterns. Subsequently we completed laboratory studies of potash recovery from brine and desulfation of brine and sea water.
In assessing the minerals potential of the sea, we did not overlook uranium, silver, and gold, metals that would not present the marketing problem of the plentiful components, such as sodium, magnesium, and chlorine. At the reported concentrations, that we believed authentic, of uranium, 3,000 parts per trillion, silver 40 parts per trillion, and gold 4 parts per trillion the economic recovery of these metals, singly or in combination, appeared hopeless. At market prices of $8 a pound for uranium, $2 an ounce for silver, and $35 an ounce for gold, the respective values are 0.005, 0.0003, and 0.0004 cents per ton of sea water. The value of the uranium is seven times more than the silver and gold combined. But, even if all the uranium could be extracted, 200 tons of sea water would have to be processed to recover 1 cent worth. In contrast, the value of some other components of sea water at nominal prices of $3 a ton for NaCl, $60 a ton for MgO, $40 a ton for sulfur, and $0.30 a pound for bromine are $0.09, $0.12, $0.04, and $0.04 worth of NaCl, MgO, sulfur, and bromine, respectively, per ton of sea water.
Action last year by international monetary authorities resulted in a dual price system for gold and focused public attention on gold supply. The lay public and some members of the technical community as well hastened to suggest to the Government that the shortage of gold could be easily remedied by tapping the ocean’s “well known” gold resources. A concentration of gold in sea water of 65,000 parts per trillion was reported in 1872, and is still frequently cited as an authentic value. This is equivalent to $0.065 per short ton and, if valid, would indeed offer a chance for profitable exploitation. When we found that the Bureau of Mines 1965 Edition of Mineral Facts and Problems gave the gold content of sea water as 130 to 260 tons per cubic mile, equivalent to 6,500 and 13,000 parts per trillion as reported by Liversage in 1896, some experiments on our part to determine the limiting value were indicated.
This paper discusses historical variations in reported amounts of gold in sea water and describes our brief experiments to resolve these differences.
The Trend of Gold Determinations in Sea Water
Marcet, writing in France in 1822, drew attention to the probable presence of trace elements dissolved in the oceans. No trace elements had been positively identified at that time. Iodine was detected in 1825, and quantitatively determined in 1852. A determination, incidentally, 200 times lower than the value now accepted. Other determinations for iodine in 1855 gave concentrations 200 times higher than the value now accepted. This tells something about the uncertainties in trace element analyses. Silver was determined in 1850, arsenic in 1851, lithium in 1855. Not until 1872, did Sonstadt report the gold content of sea water as 65,000 micrograms per cubic meter.
Twenty years later, Munster, in Norway, evaporated sea water to dryness and assayed the salts. His results showed 5,000-to 6,000 micrograms of gold per cubic meter (parts per trillion), down tenfold from Sonstadt’s determination. Ten years later, in 1902, Munster’s analysis was confirmed by Arrhenius. Starting in 1918, Fritz Haber, who was instrumental in developing German’s synthetic nitrogen compound industry, attempted to restore Post War I Germany to economic health by recovering gold from sea water. First, he bubbled SO2 through synthetic sea water spiked with gold. This reduced and precipitated about 35 percent of the added gold. The method he finally developed was to add lead acetate and ammonium sulfide to a freshly taken sea water sample. Fresh samples were used to avoid errors through adsorption of gold on the vessel walls. The lead sulfide that formed acted as a collector for the gold. To avoid possible redissolution of the gold, the precipitate was quickly separated from the solution. Lead formate and boric acid were added, and the gold determined by fire assay. As Haber became more exacting and painstaking in his work, his results became lower and lower, shrinking from thousands of micrograms per cubic meter to hundreds, then tens, and finally ending up after 10 years work at 4 micrograms per cubic meter. Haber concluded that the deceptively high values of, the beginning work were unreal and were caused by the gold content of reagents and cupels used in gold assaying.
In his decade of work, Haber analyzed 2,000 or more samples in compiling the 4 microgram per cubic meter average. With regard to regional differences, he regarded the South Atlantic as a gold-poor area of lower than average content, whereas the waters off Iceland and Greenland contained 10 times as much gold as the average. He also found that individual samples taken almost simultaneously at the same location gave widely variable results. From this he concluded that in addition to dissolved gold, some was present as colloidal particles or adsorbed in suspended particulate matter.
A summary of some patents from several countries on methods for recovering gold from sea water was given in a paper by Caldwell in 1938. These include processes for the filtration of sea water through charcoal; atomization of mercury through sea water to collect the gold; electrolytic reduction; and addition of a soluble copper or lead salt, followed by sodium or hydrogen sulfide. Caldwell went on to describe experiments he made at Oregon State University to determine the amount of gold in sea water. To a 40-liter sample he added first mercuric chloride, then magnesium metal powder and hydrochloric acid. Nascent hydrogen reduced the mercuric chloride to form a colloidal mixture of elemental mercury and mercurous chloride. As this mixture settled, it collected the gold for determination by fire assay. Caldwell did not engage in the elaborate purification of reagents and cupels that Haber did, and merely concluded that his determinations of gold at 100 to 200 parts per trillion were an upper limit.
Brooks in 1960, used an anion resin column to obtain an enrichment factor of 2 x 10 7. He acidified the sea water with HCl to 0.1 N, then passed 250 liters through a 0.5 cm x 13.2 cm column of IRA-400 resin at 2 ml per minute. The gold was so strongly adsorbed that the resin had to be ashed for analysis. He obtained 9 micrograms of gold per cubic meter of sea water.
Hummel in 1957, Oka in 1964, and Schutz in 1963, used neutron activation for analyzing sea water. Hummel irradiated sea water samples from the Bay of Biscay without any preconcentration. He reported 10 to 409 parts per trillion. Oka preconcentrated water taken off the coast of Japan, and reported 6 to 429 parts per trillion. The variations were attributed to differences in near shore and open sea samples, with the near shore samples containing more gold. Schutz preconcentrated his samples by freeze drying. His samples from the Atlantic Ocean contained 4 to 27 parts per trillion, and averaged 11 parts per trillion. Schutz found no clear pattern of regional differences.
Solubility of Metallic Gold in Sea Water
A first step towards determining whether gold was indeed present in sea water would be to determine whether metallic gold would dissolve in natural sea water. To avoid the difficulties in analyzing minute concentrations of gold, we used a radioisotope method to measure gold solubility.
Our procedure was to electroplate natural gold tagged with radioactive gold-195 on a platinum wire, expose the wire to sea water, and monitor the transfer of radioactive gold to the sea water. Sea water for our work came from a sample taken from the open sea off the coast of southern California by the Bureau of Mines oceanographic vessel R/V Cripple Creek.
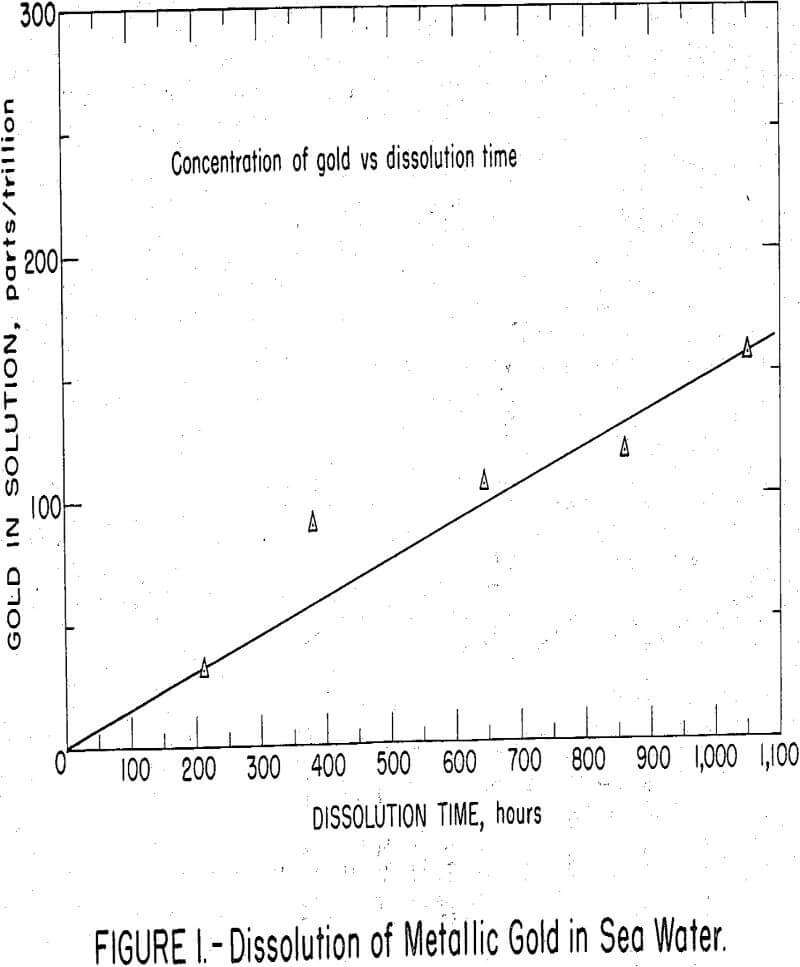
A total of 8.6 micrograms of gold was plated on a platinum wire from an AuCl3 solution containing 10 micrograms gold-198 and spiked with 20 micro-curies of gold-195, weighing 0.0005 micrograms. In the plated gold, 908,000 gamma counts per minute were equivalent to 1 microgram of gold. The plated wire was suspended in 50 ml of sea water in a stoppered and stirred beaker. At weekly intervals, a 5 aliquot was removed for counting, then returned to the beaker. At the end of 6 weeks when the test was terminated, the solution had dissolved gold equivalent to 150 parts per trillion.
As shown in figure 1, gold dissolved at a uniform rate of about 25 parts per trillion per week. The test served to, demonstrate that metallic gold does dissolve in sea water at a measureable rate. As gold trichloride is much more soluble than gold monochloride, oxidizing conditions can be assumed to favor dissolution of the gold. Peshchevitskii has calculated the solubility of monovalent gold in the form of AuCl2 in sea water as 30 parts per billion, a level about 3,000 times higher than that ordinarily found in sea water.
Sea gold cannot be recovered with gold trommels.
To obtain the gold originally in solution, we now had to analyze the initial sea water sample.
Analysis of Gold in Sea Water
The analytical procedure we elected to use was to preconcentrate the gold in solution several thousandfold by resin ion exchange or solvent extraction and determine the gold in the concentrated solution by atomic absorption spectrophotometry. Radioactive gold-195 was added to the sea water to permit monitoring the gold through the preconcentration and analytical steps.
Exploratory tests of preconcentration were made first using an anion resin, Dowex-1, in the chloride form. Four liter samples of natural and acidified sea water, each spiked with 1 mg per liter (1 part per million) of gold and containing radioactive gold, were passed downflow through 10 ml of resin in a 1 cm diameter column. At a flow rate of 10 ml per minute, 75 percent of the gold was absorbed at the natural pH of 7.8 and 99 percent was absorbed from the solution acidified to pH 2 with HCl. Even so powerful an elution solution as 2 molar sodium perchlorate removed only part of the loaded gold from the resin. This was not considered defeating as the resin could be ashed for analysis.
When an attempt was made to put acidified sea water, spiked with only radioactive gold through a 10-ml resin column at a flow rate of 10 ml per minute, pronounced leakage of gold (approximately 30 percent) through the resin occurred. As the slower flow rates, that might have avoided the leakage, would have involved many weeks more time than we were prepared to spend on the experiment, use of resins for preconcentration was abandoned.
Solvent extraction of the gold with methyl isobutyl ketone (MIBK) followed by evaporation of the MIBK to small volume for direct measurement by atomic absorption proved rapid and effective. Different volumes of sea water and MIBK were used in a studied attempt to minimize bias.
The first determination used 30 gallons (113.6 liters) of sea water. At 1.025 sp gr, the sample weighed 116 kg. Radioactive gold-195, equivalent to 0.3 microcurie and 0.0007 part per trillion by weight, was added as a tracer. This was followed by 1,080 ml of 35 percent HCl and 114 ml of 48 percent by weight hydrobromic acid fortified with bromine. The bromine and acid assured the oxidation of all gold to the auric valence and the formation of ketone extractable bromaurate ion. After blending, the solution was extracted with three 2.5-liter portions of MIBK. As the solubility of MIBK in sea water was about 20 ml per liter, only 5.1 liters of the organic extractant was collected.
The organic was evaporated in an open dish under a hot air sweep to 30 ml. Gamma counting showed 91 percent of the gold was in the concentrated MIBK extract. A 15 ml portion was submitted for analysis by atomic absorption. Part of this volume, 4.7 ml, was expended in the analysis. The residual 10.3 ml was combined with the second 15 ml portion, and the 25.3 ml so obtained further evaporated to 9 ml and then analyzed. The assays checked at 10 parts gold per trillion parts sea water. Because only 91 percent of the gold was extracted, as shown by the tracer, the value was adjusted to 11 parts per trillion.
To determine a “blank” value for the reagents used, an additional 1,080 ml HCl and 114 ml HBr-Br2 mixture was added to the depleted sea water from the first determination. The solution was then extracted with 5.1 liters of MIBK, and the organic evaporated to 15 ml for analysis. Counting of the organic showed it had extracted an additional 2.5 percent of the gold originally in the sea water. When this amount was subtracted, the “blank” was equivalent to 1 part of gold per trillion, and the corrected gold content of the sea water was 10 parts per trillion.
In the second determination, 40 gallons (151.4 liters) of sea water and proportionately more reagents were used. The organic extract was evaporated to 15 ml for analysis. Counting showed the extract contained 94 percent of the gold. Adjustment of the Atomic Absorption analysis to account for the blank and the 94 percent extraction resulted in finding 13 parts gold per trillion of sea water.
Our findings of 10 to 13 parts per trillion for gold in water from the open sea by a solvent extraction procedure seems consistent with analyses obtained by ion exchange and neutron activation methods.
Outlook for Gold from Sea Water
At a concentration of about 11 parts per trillion, a ton of sea water contains 0.001 cent worth of gold. Precipitation methods that can be postulated would use many times a thousandth of a cent worth of reagents per ton of water. Electrolytic reduction processes require electricity and capital plant, neither of which are compatible with the minute value of gold in sea water.
In any solvent extraction process, solvent losses would be many times the value of the gold. As an illustration, a very low organic loss based on industrial solvent extraction experience is 0.1 gallon per 1,000 gallons of aqueous feed solution. If an organic extractant for gold cost $0.30 a gallon, the minimum loss by solubility and entrainment would be equivalent to $0.03 worth of organic in recovering 0.004 cent worth of gold.
Ion exchange resins that we know today load gold slowly and incompletely from natural sea water. Also, they are expensive. Even if the tides could be harnessed to contact sea water with ion exchange resins, the capital cost of the resin in terms of gold that might be recovered is prohibitive. A look at the technical and economic factors in one ion exchange system often proposed for obtaining gold from sea water could be instructive. In this system, the forward motion of a ship causes sea water to flow through a tankful of ion exchange resins in the hold. Assume the tank is 100 square feet in cross section and 10 feet long, thus containing 1,000 cubic feet of resin. Industrial operations show the maximum flow rate of clarified solution through such a column is about 20 gallons per square foot per minute. At this flow rate, a total of 2,880,000 gallons or 12,500 tons of sea water would pass through the column each day, and about $0.15 worth of gold recovered. In one year of uninterrupted sailing, about $55 worth of gold would be absorbed. The 1,000 cubic feet of resin would cost about $50,000 and the resin life might be 5 years for an annual resin cost, independent of interest charges, of $10,000 a year. The potential $55 worth of gold a year is inconsequential in this context.
Even if regional differences in the gold content of the oceans should reveal localities containing gold at 10 or even 50 times the 10 parts per trillion level, increased value of 0.05 cent per ton still falls far short of that needed for economic exploitation.
Summary
In summary, the reported concentration of gold in sea water that has been generally accepted decreased from 65,000 parts per trillion in 1872 to 4 parts per trillion in 1928. There it remained until confirmed neutron activation analysis established a level of about 11 parts per trillion in 1964. Our own determinations using radiotracers to check on a solvent extraction-atomic absorption method confirms the 11 parts per trillion analysis. Further, by radiotracer technique we established that elemental gold does dissolve in sea water at a measureable rate. (Sample Splitter)
We know of no procedure now, nor do we see anything on the horizon, that can recover gold economically from sea water.
You might like: 8 Best Whole House Water Filters
