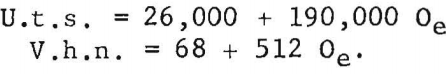Table of Contents
The purpose of this investigation was to determine the effects of the impurities, oxygen, nitrogen, carbon, and iron (in low percentages) on the mechanical properties of electrolytic titanium. The strength and hardness of binary Ti-O, Ti-N, and Ti-C alloys are linear functions of alloy content in the composition range investigated. Compared to oxygen and carbon, nitrogen is the most effective strengthener and hardener, followed by oxygen, then carbon. When the carbon concentration is above 0.10 percent, carbides are present, and strength and hardness are relatively unchanged.
For equivalent hardness levels, Ti-Fe alloys are stronger than binary titanium alloys containing oxygen, nitrogen, or carbon. However the strengthening is related to the presence of transformed beta in the structure.
The major impurities in commercial titanium are iron, manganese, silicon, oxygen, nitrogen, carbon, and hydrogen. Although the effects of small quantities of these impurities on the properties of titanium and its alloys have been the concern of numerous studies a complete understanding of their individual and collective effects is not known. The interstitial solutes oxygen, nitrogen, carbon, and hydrogen are likely to be picked up by titanium in all processing operations and have been shown to have large effects on its properties. The Bureau of Mines reported on the relative effects of oxygen, nitrogen, carbon, and iron on the Brinell hardness of electrolytic titanium.
The availability of high-purity, low hardness (60 to 80 Brinell hardness number, B.h.n.) titanium resulting from fused-salt electrorefining research permitted a reassessment and evaluation of the mechanical properties of titanium and its alloys. Despite the low level of impurities in electrorefined titanium, it is not a pure metal. This study, in essence, is an attempt to isolate the contribution of individual contaminants on the mechanical properties of dilute titanium alloys.
Experimental Procedure
The preparation of alloys, description of melting equipment, melting techniques, and Brinell hardness testing procedures for titanium used in this investigation have been described.
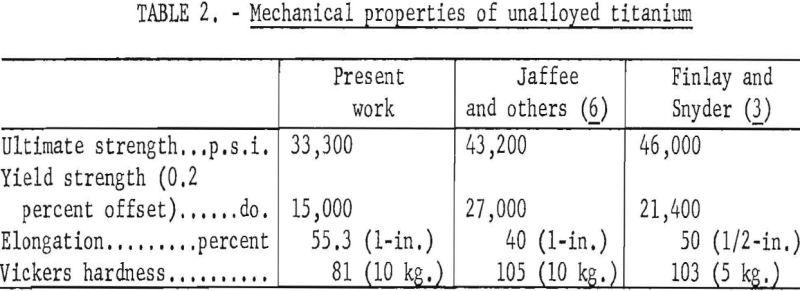
Alloys used in this study were arc-melted ingots remaining from the hardness test work of Haver and Baker. These alloys were prepared by adding measured amounts of reagent grade titanium dioxide, titanium nitride, carbon black, and iron powders to composite samples of (73 B.h.n,) electrorefined titanium.
Arc-melted alloys of compositions shown in table 1 covered a hardness range of 78 to 158 B.h.n. The button-ingots were machined and sanded on both sides to give smooth and parallel surfaces, and cold-rolled into sheets one-sixteenth-inch thick. An anneal of 30 minutes at 700° C. in vacuum preceded the final reduction of 65 percent.
Specimens for microscopic examination, hardness, and tensile tests were annealed in a vacuum of 10 -4 to 10 -5 millimeters of mercury for 30 minutes at 700° C. and were allowed to cool in an atmosphere of helium. Duplicate specimens of longitudinal sections were examined metallographically, and 5 to 7 Vickers hardness measurements were taken using a 10-kilogram load. Microstructures were developed with an aqueous solution of 1.5-percent hydrofluoric acid and 3-percent nitric acid.
Tensile specimens were prepared with a 1-inch gage length and a width of one-quarter inch in the reduced section. Tensile tests (4 to 6 per alloy) were conducted at a crosshead speed of 0.025 inch per minute to the 0.2 per cent offset yield strength, and at 0.050 inch per minute thereafter.
Discussion and Results
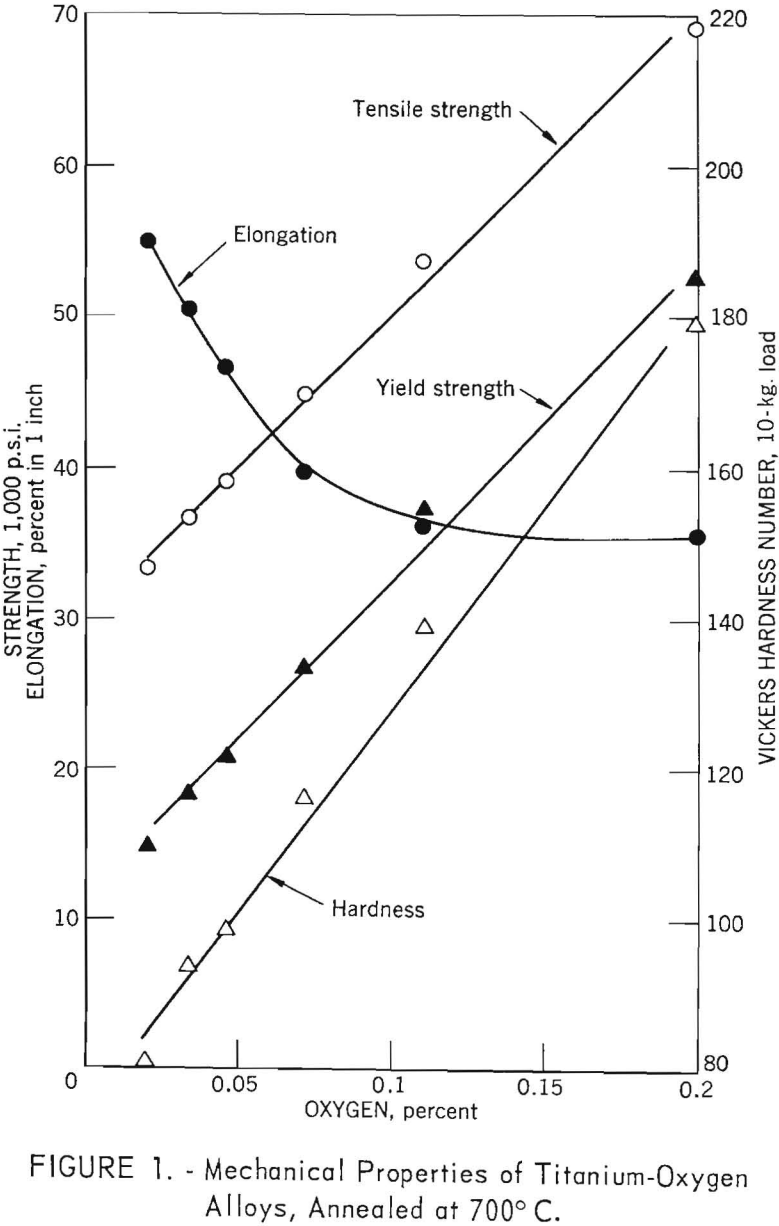
The mechanical properties of unalloyed electrorefined titanium (average of 12 specimens) are shown in table 2, together with average values found for iodide titanium, by Jaffee and other and Finlay and Snyder. The lower strength and hardness for electrorefined titanium are attributed to the lower impurity concentration. The reported concentrations of carbon and iron in weight-percent, for the iodide metal, were 0.03 carbon and <0.04 iron, compared to 0.015 carbon and <0.005 iron for the electrorefined metal.
The mechanical properties of electrorefined titanium as a function of oxygen content are shown in figure 1. Strength and Vickers hardness are straight line functions (determined by the “least squares” method) of oxygen content, as oxygen content is increased from 0.021 to 0.20 weight-percent. Tensile strength increases from 33,300 to 69,300 pounds per square inch, while hardness increases from 81 to 179 Vickers hardness number (V.h.n.). Ductility, as measured by tensile elongation, shows a decrease of approximately 34 percent for oxygen contents up to 0.11 weight-percent, and appears
to be relatively constant up to 0.20 weight-percent oxygen, the maximum percentage investigated.
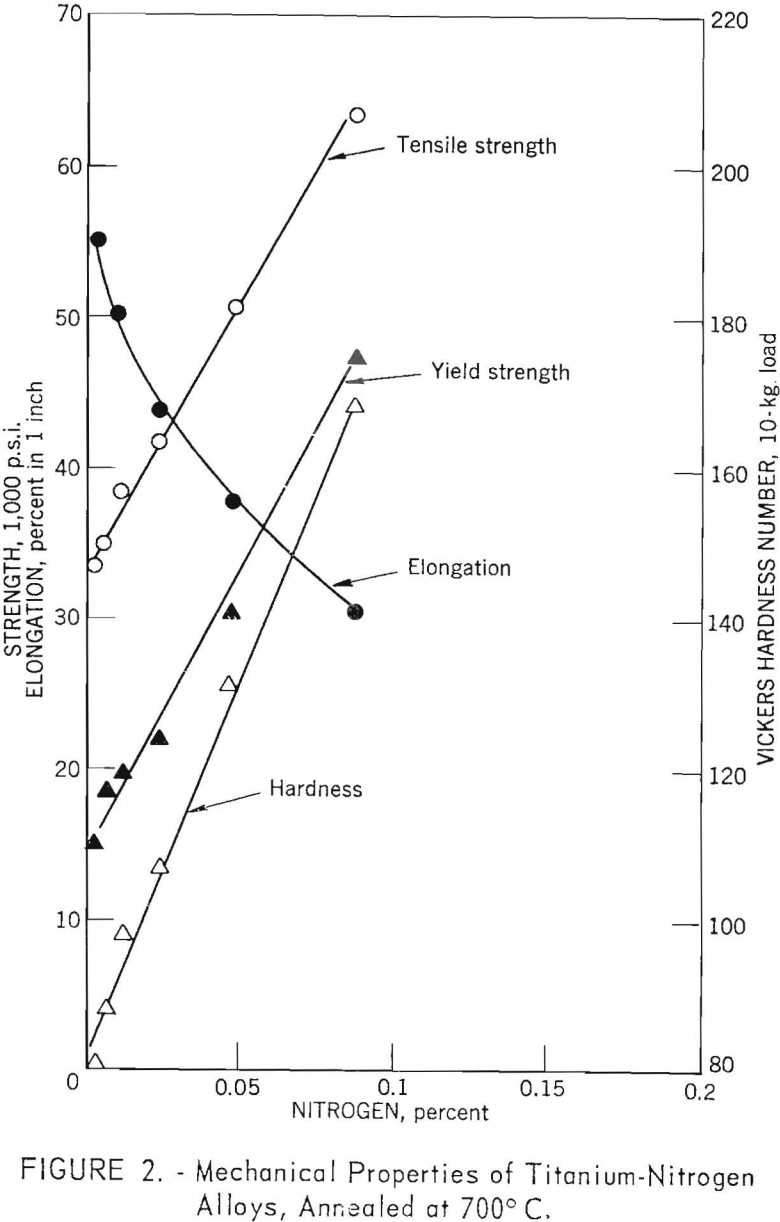
The strength and hardness versus composition curves for titanium-nitrogen alloys (fig. 2) are similar to those for oxygen; however, hardening and strengthening rates for nitrogen are greater than those for oxygen. For the range of nitrogen contents investigated (0.004 to 0.087 weight-percent) strength, hardness, and ductility changes were similar to those for oxygen in the range of 0.021 to 0.20 weight-percent; however, the addition of nitrogen caused a sharp decrease in ductility, and there was no evidence that the curve would level off.
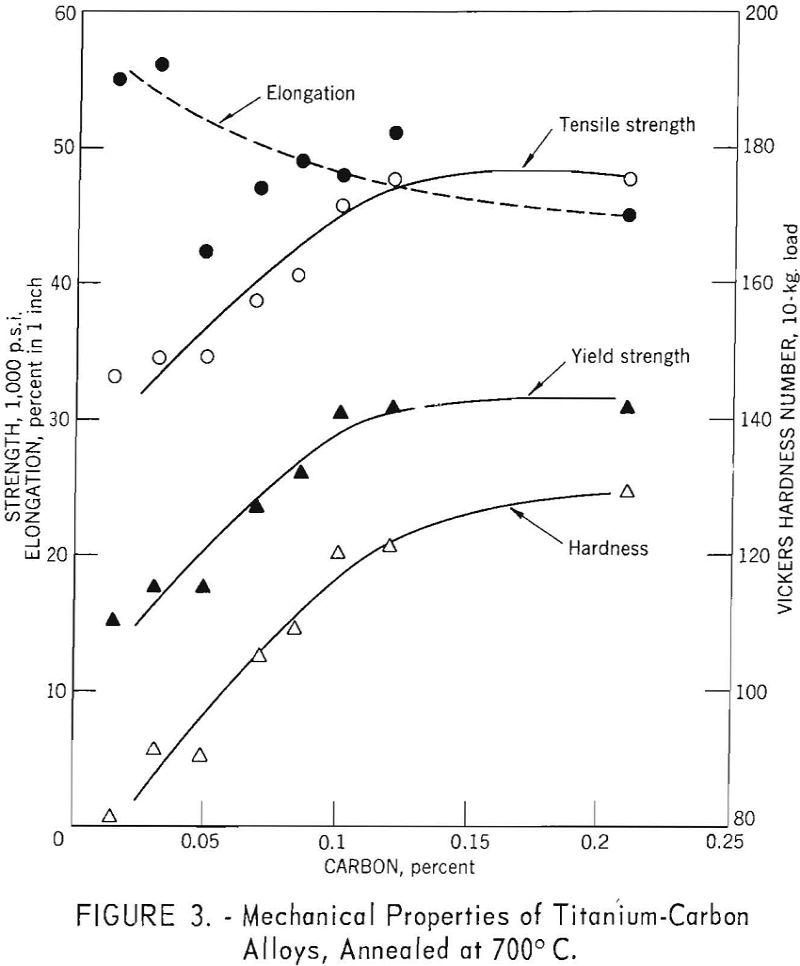
The mechanical property versus composition curves for titanium-carbon alloys are shown in figure 3, for carbon concentrations up to 0.21 weight-percent. Strength and hardness increases are essentially linear up to 0.10 weight-percent carbon; above this value carbides are present and, while hardness increases slightly, there is no increase in strength. Elongation values were erratic, but the general trend was for decreasing ductility with increasing strength.
Mechanical properties of the titanium-iron alloys are parabolic functions of iron content (fig. 4). While iron appears to be a more effective strengthener of titanium than carbon, solubility limits and microstructure changes preclude direct comparison with the interstitially soluble elements.
The solubility of iron in alpha titanium is less than 0.2 percent at the eutectoid temperature of 590° C. The eutectoid transformation is very sluggish, and only after prolonged heat treatment is TiFe found. The solubility of iron in beta titanium is approximately 25 percent at the eutectic temperature of 1,085° C.
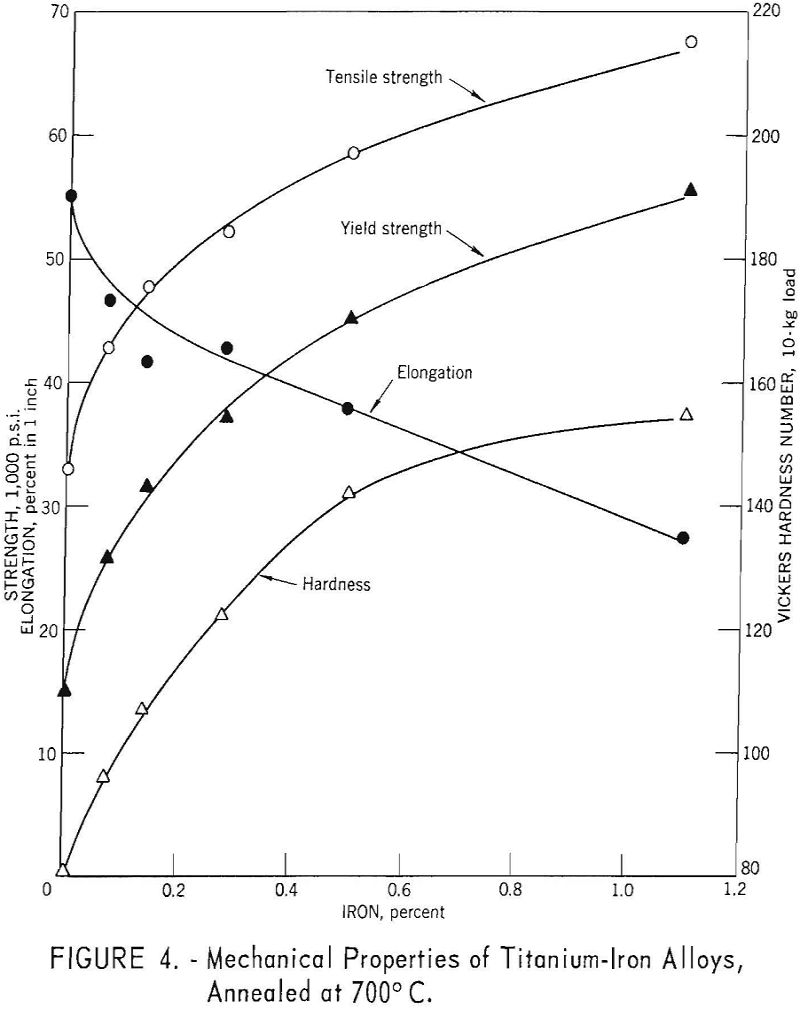
More than 3.8 weight-percent iron is required to retain the beta structure upon quenching. However, during rapid cooling from temperatures above 800° C., the beta phase in alloys containing less iron than the amount required to retain beta, may transform to acicular alpha, of the same alloy content as the parent beta phase.
McKinsey and Martin have determined that the effect of iron on the tensile properties of sodium-reduced titanium, after a variety of heat treatments, was related to quantity, distribution, and iron content of retained or transformed beta in the structure. The composition of the sodium-reduced base metal (weight percent) was reported to be: 0.04 iron, 0.047 carbon, 0.13 oxygen, 0.007 nitrogen, and 0.012 hydrogen. The effects of iron were established by eliminating the contribution of oxygen, nitrogen, and carbon using the methods outlined by Ogden and Jaffee.
Ogden and Jaffee have shown that the interstitially soluble elements-oxygen, nitrogen, and carbon-are additive in commercial grades of titanium. This is also true for electrorefined titanium; however, hardness levels for high-purity, low-hardness, electrorefined titanium have been found to be lower than those calculated from oxygen equivalents. In the present work, data for oxygen, nitrogen, and carbon (up to 0.12 weight-percent carbon), added singly to electrorefined titanium, fit a straight line (fig. 5) having the equation:
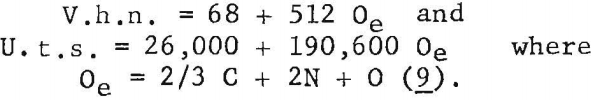
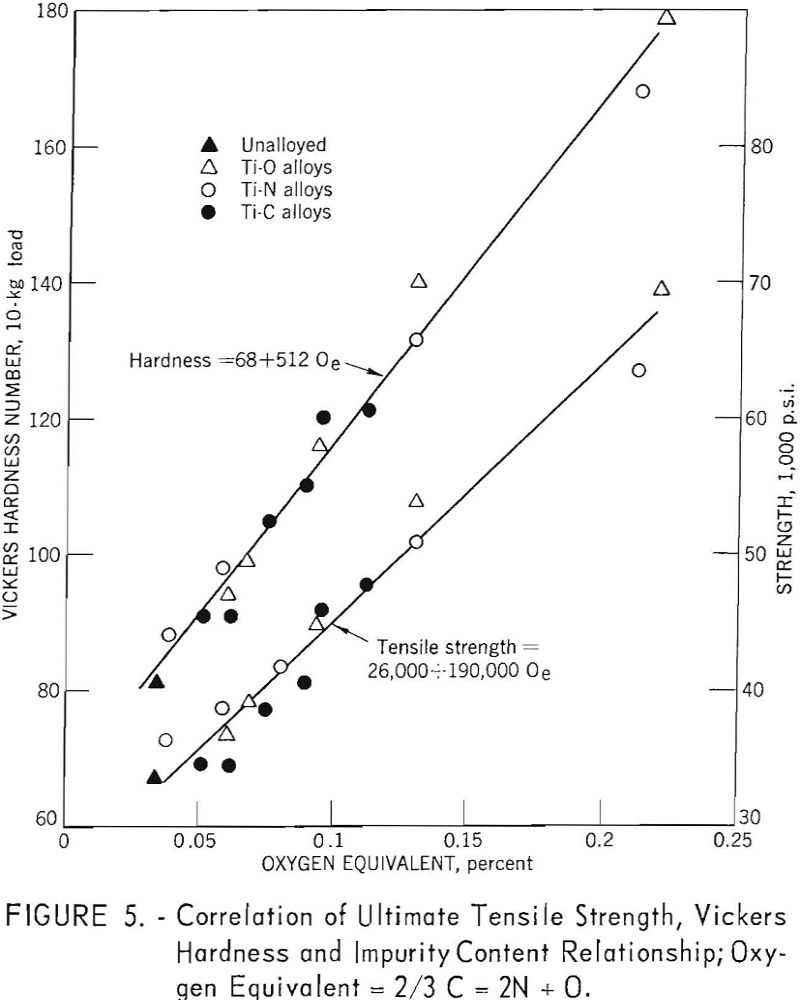
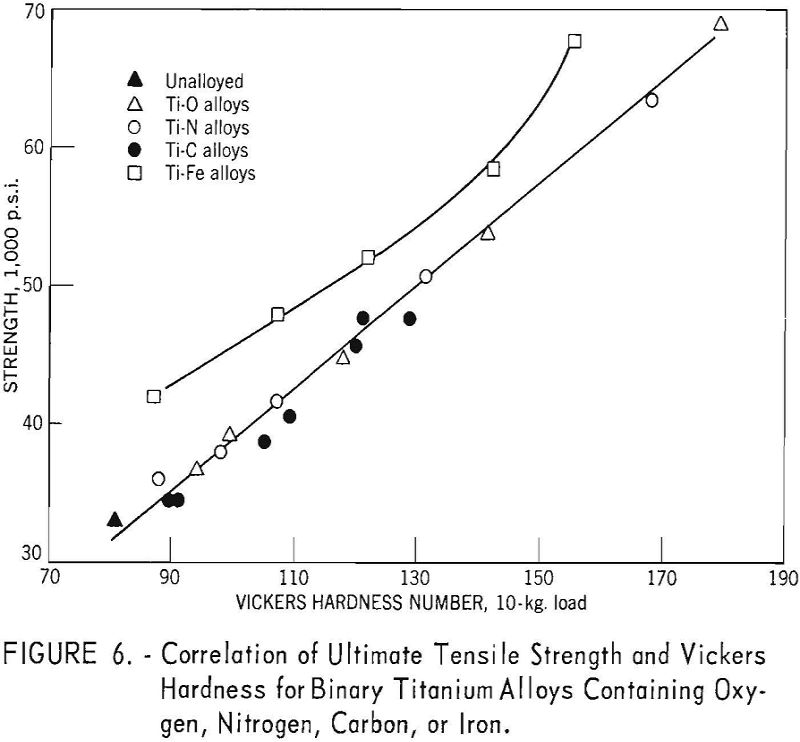
The correlation between Vickers hardness and ultimate tensile strength is shown in figure 6. The data for annealed oxygen, nitrogen, and carbon alloys fits a straight line having the equation:
U.t.s. = 1,800 + 370 V.h.n.
For equivalent hardness, the strength levels of titanium-iron alloys are from 5,000 to 8,000 pounds per square inch greater than for the oxygen, nitrogen, and carbon alloys.
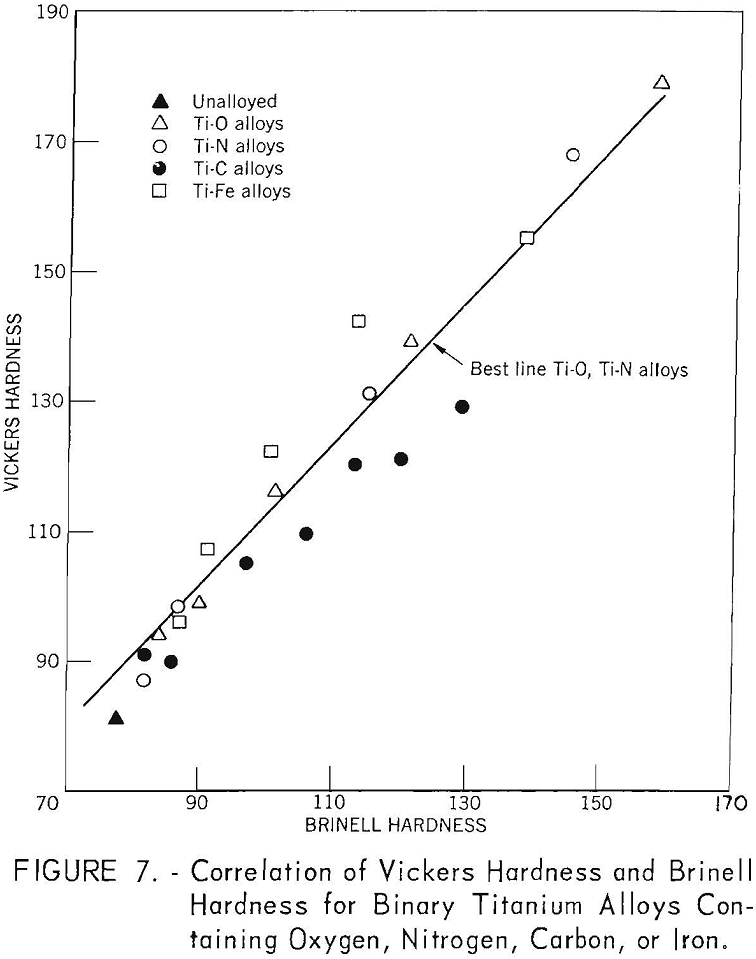
The correlation of Brinell hardness with Vickers hardness is shown in figure 7. There is a very good correlation for titanium alloys containing oxygen and nitrogen, but considerable deviation is noted for titanium-iron and titanium-carbon alloys.
Because of initial differences in purity and properties, of the base titanium metals used in this study, and that of Finlay and Snyder, and Jaffee and others, their mechanical property data could not be extended to the lower impurity level of alloys of this investigation. However, it is
noted at the 0.1 weight percent impurity level, strengths of electrorefined titanium base Ti-C, Ti-O, and Ti-N alloys are approximately 10,000 pounds per square inch lower than iodide titanium-base alloys. Elongation values are in good agreement with reported values of Finlay and Snyder, who cold-rolled their alloys and annealed them for 1 hour at 700° C. Strength and elongation values for titanium-iron alloys are in very good agreement with the work of Finlay and Snyder. However, hardness values for titanium-iron alloys based on electrorefined titanium are 15 to 20 Vickers points lower than those for iodide titanium-base alloys.
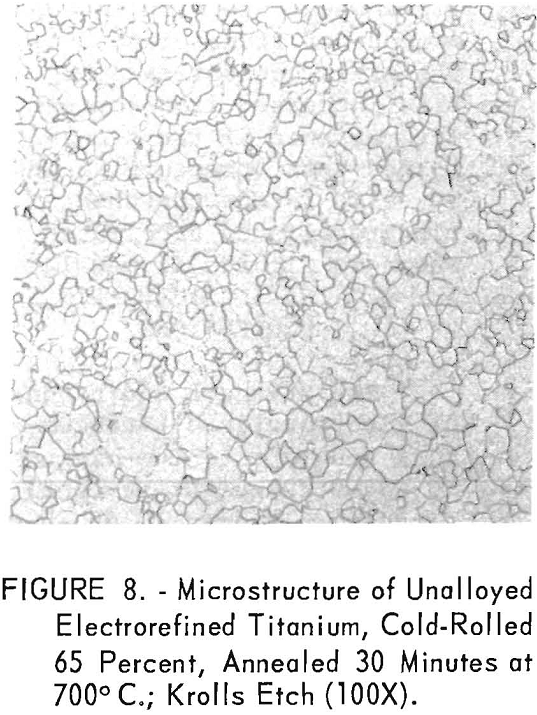
The microstructures of unalloyed electrorefined titanium, cold-rolled from the arc-melted condition and annealed 30 minutes at 700° C., exhibit the typical equiaxed alpha grain structure and are shown in figure 8.
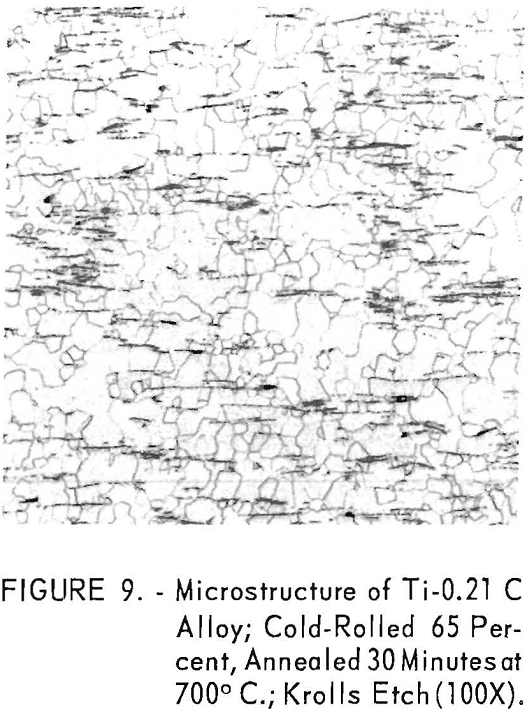
The first presence of the carbide phase in titanium-carbon alloys is evident at 0.12 weight-percent carbon and is in fair agreement with solubility limits of 0.12 weight-percent at 600° C. reported by Cadoff and Nielsen. The 0.21 weight-percent carbon alloy (fig. 9) exhibited extensive amounts of the carbide phase, orientated in the rolling direction.
Oxygen and nitrogen are both soluble in alpha titanium for the compositions investigated, and the microstructures of the binary titanium-oxygen and titanium-nitrogen alloys showed only equiaxed alpha grain structures.
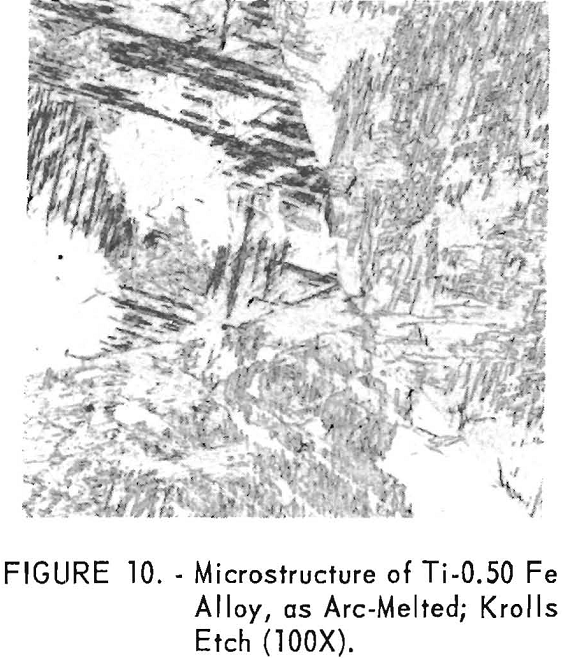
The structures of arc-melted titanium-iron alloys, of this investigation, were typical acicular structures for iron contents greater than 0.07 weight-percent (fig. 10). Subsequent cold work and annealing at 700° C.
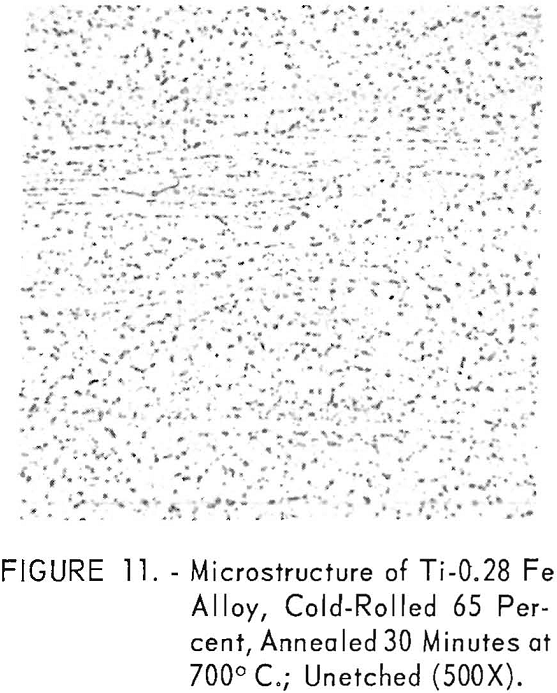
resulted in a fine-grained alpha structure with increased amounts of transformed beta in the grain boundaries, along with increased iron content. The presence of the transformed beta structure is best revealed in the unetched electropolished specimens shown in figures 11 and 12.
Conclusions
The strength and hardness of electrorefined titanium-base binary titanium-oxygen (0.034-0.20 weight-percent), titanium-nitrogen (0.006-0.087 weight-percent), and titanium-carbon (0.033-0.12 weight-percent) alloys using electrorefined titanium are linear functions of alloy content. The strength is approximately 10,000 pounds per square inch lower than values reported for iodide titanium-base alloys. Lower strength and hardness are attributed to lower initial total impurity content of the electrorefined metal. Elongation values of the electrorefined titanium-base alloys are similar to iodide titanium-base alloys similarly processed, with decreasing ductility for increasing impurity contents.
Hardening and strengthening trends are similar to those reported for iodide titanium and commercial Kroll sponge-base alloys. Compared to oxygen and carbon, nitrogen is the most effective strengthener and hardener, followed by oxygen, then carbon. In the composition range, where carbides are present (above 0.10 weight-percent carbon), strength and hardness of titanium-carbon alloys increased only slightly.
Iron appears to be a more effective strengthener of titanium than carbon, but its effects are attributed to the presence of transformed beta in the structure. For equivalent hardness levels, the strength of titanium-iron alloys are from 5,000 to 8,000 pounds per square inch greater than for binary titanium alloys with oxygen, nitrogen, or carbon.
Using the established relationship
Oe (oxygen equivalent) = 2/3 C + 2N + O………………………………..(9)
the strengthening and hardening effects of oxygen, nitrogen, and carbon (up to 0.10 weight percent carbon) are linear functions of oxygen equivalents: