Table of Contents
Pure Minerals always Have same Composition
A pure mineral, one that is not mixed with any other mineral, is always of the same composition (certain exceptions). For example, iron pyrites is composed of iron and sulphur, in the proportion of 46.67% of iron and 53.33% of sulphur; and any specimen of the pure mineral will, when analyzed, always contain iron and sulphur in these proportions. But it is usual to find iron pyrites more or less mixed with other minerals, and the analysis of an ordinary specimen will be somewhat different from that given above.
Atomic Weights and Composition Formulas
Chemists have worked out a very handy way of stating the composition of substances by what may be called composition formulas. All substances are made up of about 80 simple substances, called elements. Some substances are composed of a single element. For example, native gold, silver, copper, and sulphur are examples of minerals each of which is composed of a single element of like name. Substances made up of two or more unlike elements are called compounds, and the elements in compounds are combined in twos, threes, etc. For example, calcite, the mineral of limestone, is composed of three elements, calcium, carbon, and oxygen; hematite is composed of iron and oxygen; galena, of lead and sulphur, etc. It has also been found that the composition of minerals, as well as of all other substances, is on such a simple, natural plan that it can be stated in terms of certain numbers, called atomic weights, one number being assigned to each of the 80 or so elements. For example, the numbers (atomic weights) for lead, iron, oxygen, and sulphur are 207, 56, 16, and 32, respectively (omitting small fractions.) The composition of galena is such that the weight of the lead is to the weight of the sulphur as 207 is to 32. The composition of iron pyrites can be stated as 56 of iron to 2 x 32 of sulphur; and of hematite as 2 x 56 of iron to 3 x 16 of oxygen.
A very convenient shorthand has been built up in connection with this. A letter (or two letters) is chosen as a symbol to represent the name and the weight-number (atomic weight) of each element; thus, Pb represents 207 parts of lead (by weight), Fe = 56 parts of iron, O = 16 parts of oxygen, and S = 32 parts of sulphur. By placing these symbols together, what are called composition formulas are constructed for substances composed of two or more elements. Thus, galena has the formula PbS, which means that it is composed of lead and sulphur in the proportion of 207 to 32. The composition formula for iron pyrites is FeS2, the subscript 2 being a multiplier of the value of the symbol S. A subscript always belongs to the symbol that precedes it. Consequently, from this formula, it is known that iron pyrites is composed of iron and sulphur in the proportion of 56 parts of iron to 2 x 32 = 64 parts of sulphur. Hematite has the formula Fe2O3, which means that it is composed of 2 x 56 = 112 parts of iron and 3 x 16 = 48 parts of oxygen.
These formulas, when rightly understood, convey a great deal of information. Since only about 30 elements are represented in the composition of the common minerals, the symbols and atomic weights of these may be memorized with little effort; then, if the formula for any particular mineral be known, the percentage of each element in it can be readily calculated. Thus, to find the per cent of iron in pure hematite, which has the formula Fe2O3.
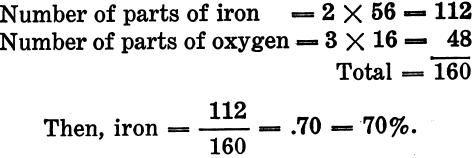
By proceeding in this manner, the per cent of any element in any mineral whose formula is known can be readily found.
At the end of this Part, a table is given that includes all the known elements, their symbols, and their atomic weights according to the latest determinations. In this table, the atomic weight of iron is given as 55.84, of sulphur as 32.064, of silicon as 28.06, etc. But for calculating the per cent of an element in a mineral, it is sufficiently exact to take iron as 56, sulphur as 32, and silicon as 28. In any case, not more than one decimal place should be used.
Chemical Changes in Minerals
The prospector should have a clear idea of the nature of certain changes that minerals often undergo, which may be so radical that minerals are transformed into other minerals; these transformations are called chemical changes. For example, when iron pyrites is acted upon by air and water, it becomes changed into the rusty substance, limonite, well known to prospectors as gossan. The pyrites, air, and water all take part in this change, and a second new substance, sulphuric acid, which is not noticed, is formed at the same time.
