Table of Contents
High-intensity wet magnetic separators have been successfully introduced into, the mineral processing field over the past ten to fifteen years, due largely to rapid advancements in magnet design. Wet magnetic separation, until recent years, was applied solely to the concentration of minerals of high magnetic susceptibility, such as magnetite, at relatively coarse sizes. Now, however, high-intensity separators are capable of treating weakly paramagnetic minerals and have extended the range of treatable particle size down to about one micrometre.
Basic Principles of Magnetic Capture
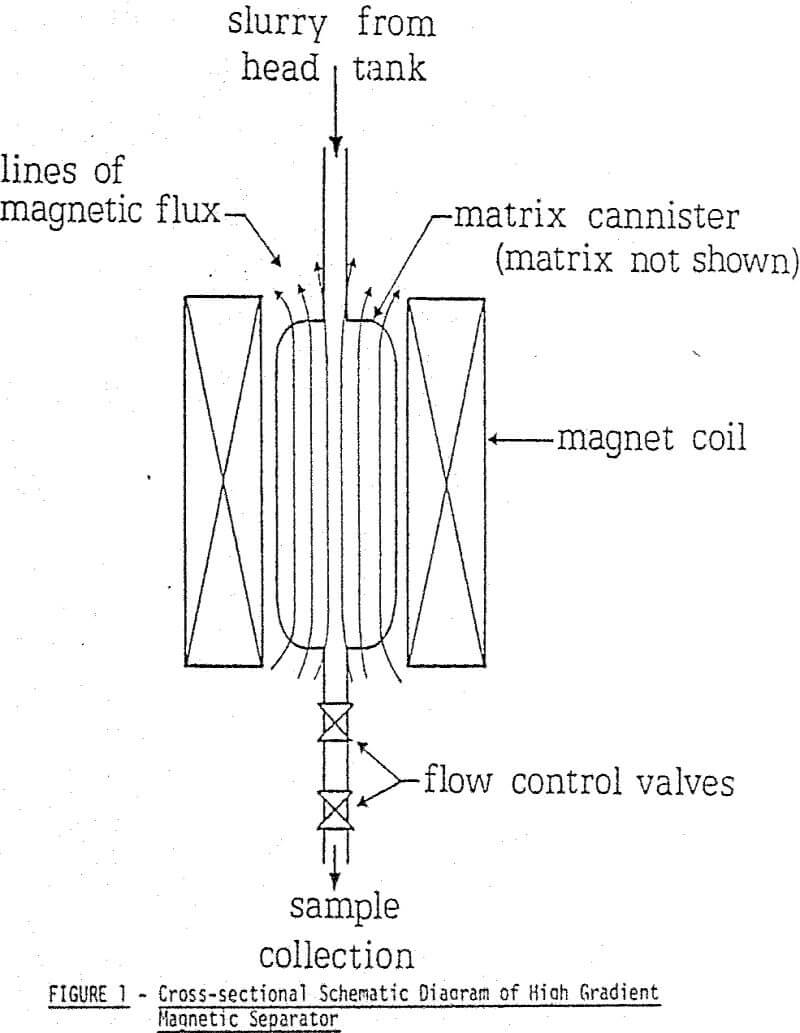
For the purpose of initially analysing the forces involved in particle capture, an idealized situation describing the separation process can be applied. A spherical paramagnetic particle in a fluid moving at constant velocity, approaches a ferromagnetic wire of circular cross section. A uniform magnetic field applied perpendicular to the wire axis magnetizes the wire and a magnetic force acting on the particle is developed. If the magnetic force is large enough to overcome the competing hydrodynamic force then the particle will adhere to the wire.
FM = V Mp dH/dX
The force is thus proportional to three terms: the volume of the particle, the particle magnetization, and the field gradient over the dimensions of the particle. A brief description of how field gradients are created in an HGHS follows.
Competing forces acting on the spherical particle include hydrodynamic drag, gravity and interparticle affects such as electrostatic attraction, friction and magnetic attraction. However, when handling particles between about 5 and 50 µm in an HGMS, the predominant competing force is hydro dynamic drag. If a Stokesian regime is assumed for the simplified case, then the drag is given by:
FD ∝ d η u
Experimental
A model capable of predicting HGMS performance from readily obtainable test data would be invaluable as an initial guide to applicability of this separation technique. For this reason an empirical model of capture was developed.
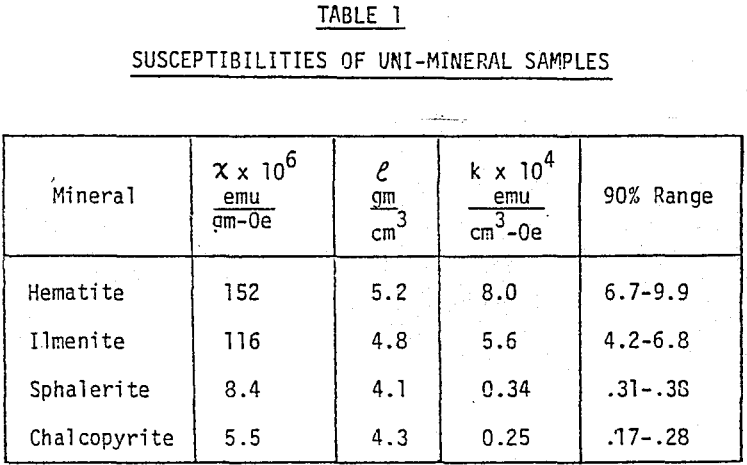
In order to closely control particle size and magnetic susceptibility, samples with narrow size and susceptibility ranges were prepared. Pure samples of hematite and ilmenite (98% Fe2O3 or FeTiO3) with narrow susceptibility ranges were obtained from mines in Quebec.
A magnetic response curve of a given material can be generated by passing the material through the Frantz several times at a set side slope and at successively higher currents. Each “non-magnetics” product becomes the feed for the succeeding pass. A plot of cumulative weight % reporting to the magnetics vs current describes the magnetic susceptibility distribution of the material.
Methodology of Evaluating Separation Performance
The size distribution of the sample is easily determined through the use of the Warman Cyclosizer, which divides the sample into five distinct size increments. Application of the magnetic susceptibility distribution is not as straight forward. As was previously demonstrated, the Frantz can be employed to produce a magnetic response curve of a mineral-bearing sample.
For the unimineral samples used in the testwork, the response curves were very steep, indicating that each sample had a narrow range of susceptibility. A sample such as a concentrate, however, will often contain several minerals, each with a distinct magnetic susceptibility. Also, because of interlocking of mineral particles, many particles will exhibit susceptibilities in between those of the individual minerals; (the net volume susceptibility of a particle containing two or more different minerals is the sum of the products of the volume susceptibility and volume fraction of each mineral). Thus, the magnetic response curve for the concentrate may have a relatively low slope with a wide range of susceptibility, a curve with no discrete increments.
The above technique can be performed on all particle size increments, since each size increment may have a unique response curve. For simplification, however, the response curves for the different size increments can be averaged so that the whole sample can be represented by a single curve.
Model Applications
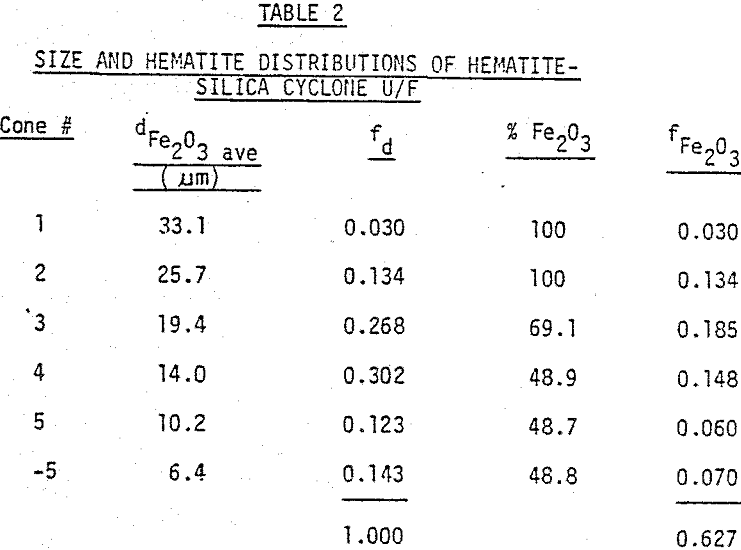
An iron ore sample from the Carol Lake deposit in Quebec was made suitable for preliminary testing of the model through the following sample preparation: (1) sequential grinding to -400 mesh (2) magnetite removal with the Davis Tube and (3) classification with a laboratory cyclone. The cyclone underflow consisted mainly of hematite and silica with a size range compatible to that which the model was developed (8 to 35 µm).
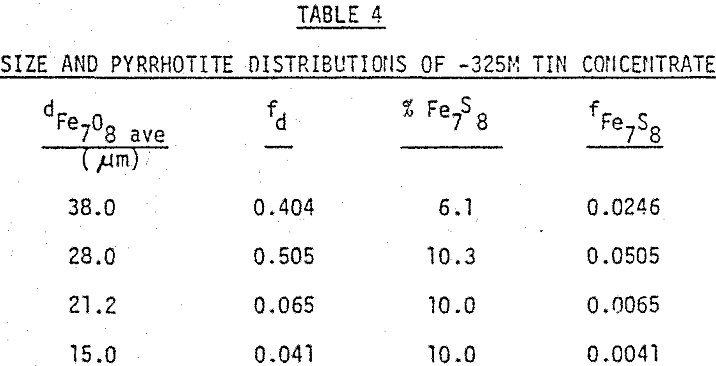
A tin concentrate containing mainly pyrrhotite (Fe7S8) and cassiterite (SnO2) was obtained from a gravity concentration circuit at the Sullivan Concentrator, Cominco Ltd, Kimberley, B.C. To produce a sample with a size distribution within the range of the model the concentrate was screened at 325 mesh.