The equipment for the separation of the rare earths consists of nearly 200 columns thirty inches in diameter by twenty feet high. They are constructed of materials which will not contaminate the rare earth products. Most of the cation exchange resin in the plant is a styrene-divinylbenzene sulfonic acid resin such as Amberlite IR-120 and Dowex 50. Included in the auxiliary equipment to the columns are precipitation tanks with covers. The solutions placed in these tanks are stirred with rubber coated agitators. The filters used for the high-purity product are ceramic, and filter cloths are used as the filtering media. The ethylenediaminetetraacetic acid (EDTA,) solutions used are prepared in equipment which controls the quantity of solid EDTA added, the amount of water needed, and the necessary ammonia required to adjust the solution to the correct pH.
The cation exchange resin used in the separation of rare earths consists of small spherical beads containing sulfonate groups attached to a styrenedivinylbenzene matrix.. The beads are extremely rugged and under reasonable operating conditions should last for many years. Cations attached to the sulfonate groups are able to come to equilibrium with cations in a surrounding solution.
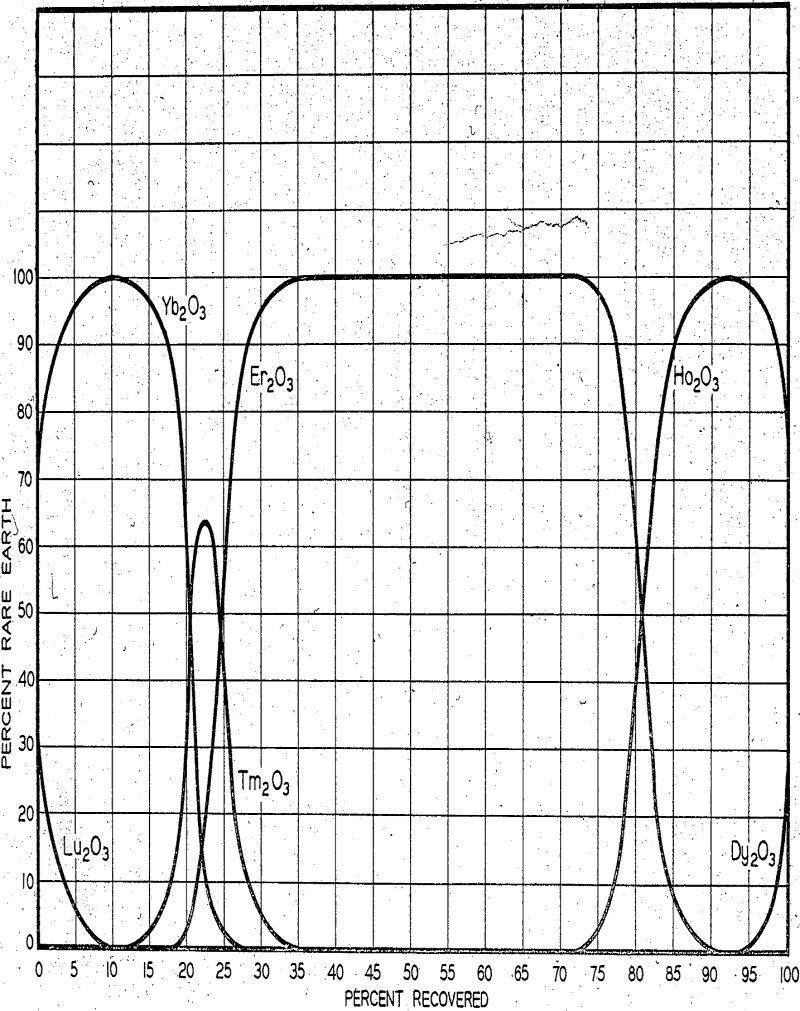
In the above manner an equilibrium is approached. The rare earths tend to arrange themselves in adjacent bands, each band containing a single rare earth. Each band moves slowly along through the columns, in position and with a speed dependent on the flow rate of the eluate. To visualize the mechanism of transport through the columns under equilibrium conditions, let us examine a band of one particular rare earth, holmium, with a band of another rare earth, dysprosium, behind and a band of a third rare earth, erbium, in front.
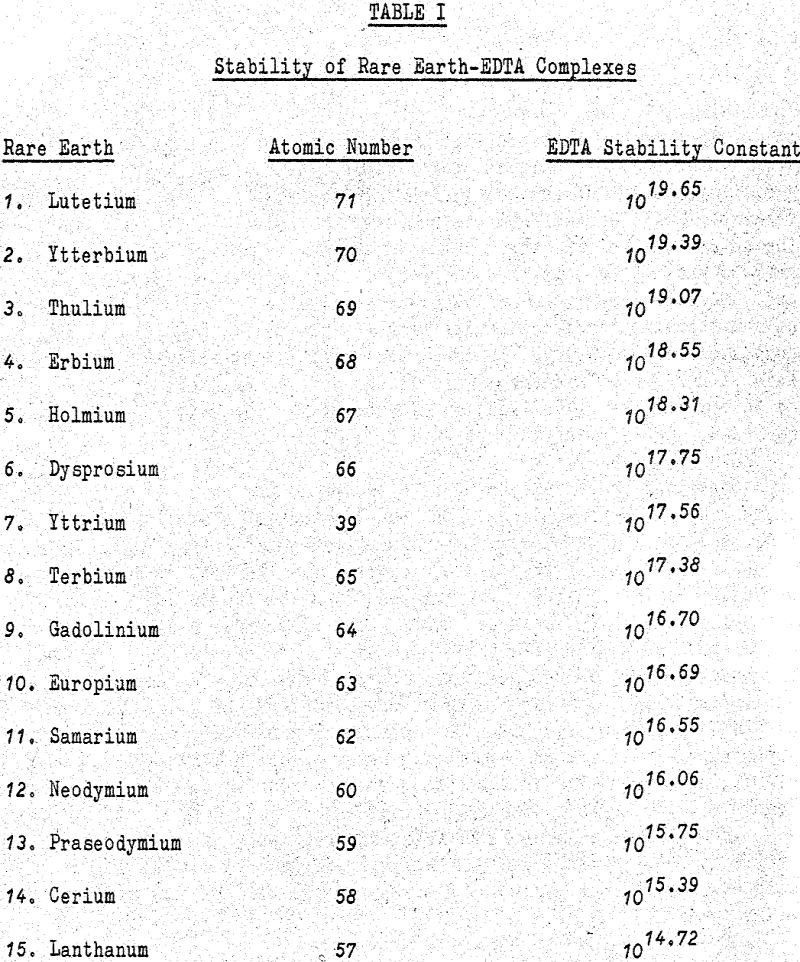
The ease of separation of any one rare earth can rougly be determined by noting the difference between the stability constants of the two adjacent rare earths. In general, the greater the difference, the easier the separation. Unfortunately the speed at which equilibrium is approached in these reactions is quite slow. As a result the flow rate of eluate to the columns must be kept low enough to allow the system to approach equilibrium.
There are other problems that should be considered. The control of the Hydrogen ion concentration should be regulated for if the acidity of the system is too high, EDTA will precipitate in the columns and cause difficulty, and if the pH is too high, the lutetium and other heavy rare earths will leak through the copper-retaining ion and be lost or a copper complex with EDTA (Cu2EDTA) will precipitate and plug up the columns and stop the flow of solution. The concentration of the eluting solution (EDTA,) must be regulated carefully or rare earth-EDTA complexes as well as EDTA and a copper complex may pre-cipitate in the columns. Flow rate must also be regulated so that the optimum yield of the highest purity material may be obtained.
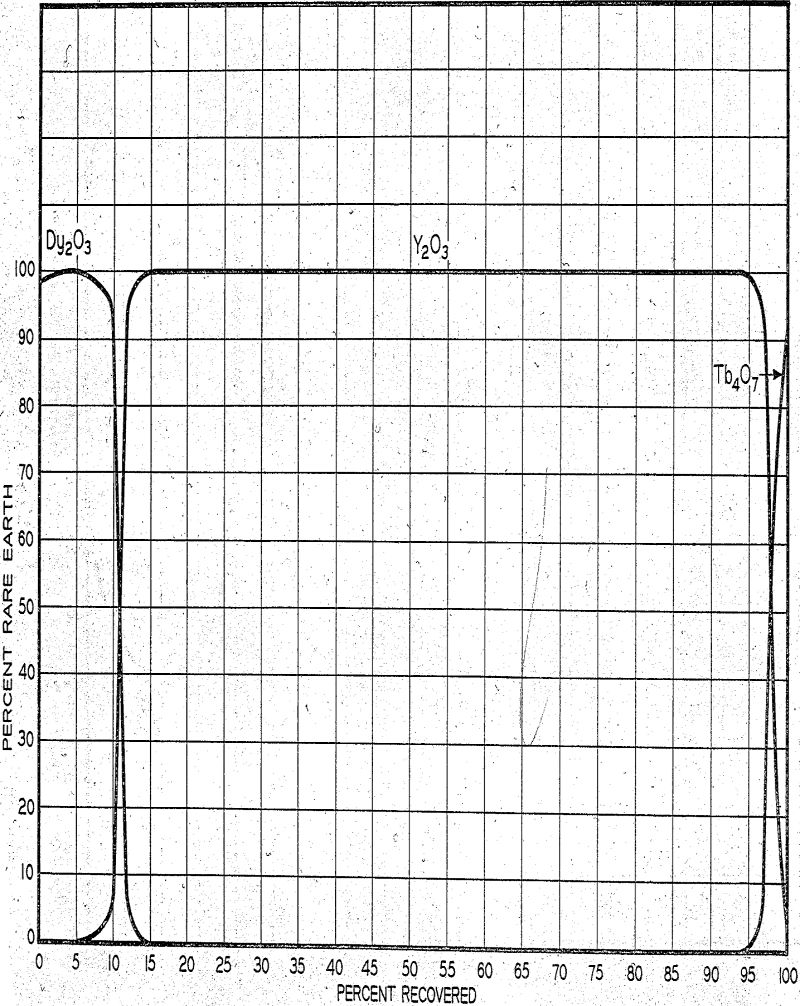
The main band of yttrium plus dysprosium and minor quantities of the other rare earths continue through a number of additional copper ion-containing columns. When separation of yttrium from dysprosium is considered sufficient, rare earth-EDTA complex is eluted off the leading column to tanks where it is precipitated with oxalic acid, filtered, dryed, and calcined.
We have produced ton quantities of the pure heavy rare earth oxides. Included in largest amounts are dysprosium oxide, erbium oxide, holmium oxide, gadolinium oxide and ytterbium oxide of 99.9% purity. Hundreds of pounds of the other less abundant rare earth oxides, such as lutetium oxide, thulium oxide, and terbium oxide also have been produced in 99.9% purity.