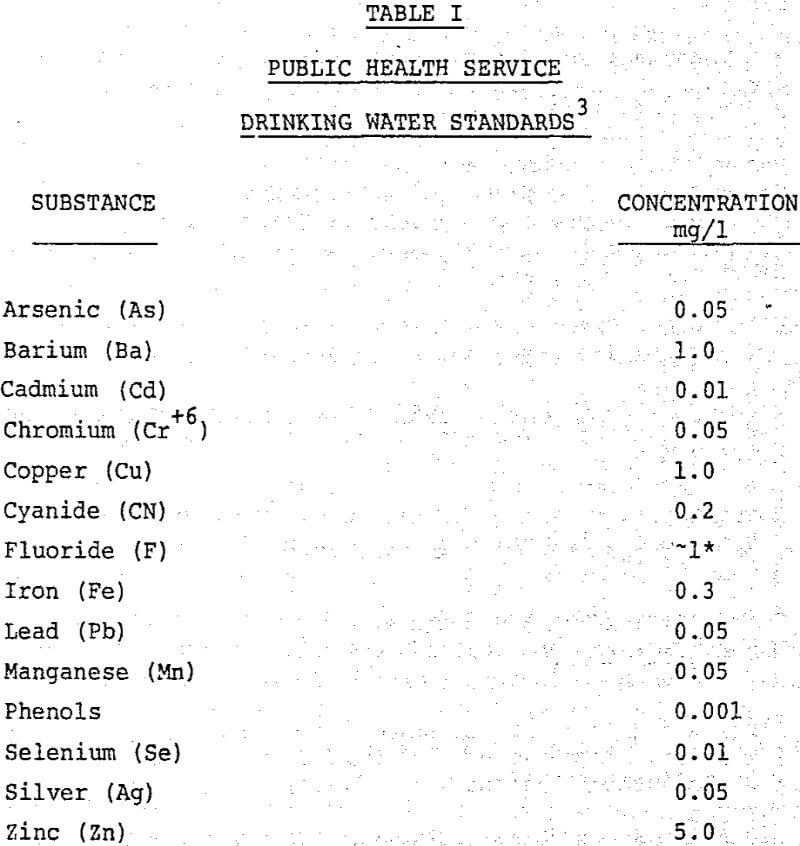
Table of Contents
The mining and processing of basic metals relies on water as a medium for transport. Metallurgical ores are ground, concentrated, leached and refined in an aqueous medium. Water is used for temperature control during processing and in air pollution abatement during smelting.
Treatment Technology
The water and wastewater treatment technology required to meet treatment requirements for water reuse or discharge are well documented in the literature. Heavy metals have been removed by oxidation, reduction, precipitation, activated carbon and chemical adsorption, ion exchange and even reverse osmosis. Some of the processes relating to heavy metal removal will be presented here.
Most soluble heavy metals are found in wastewaters as a result of contact of an acid medium with a metal in operations such as acid ore leaching, metal cleaning for finishing, or smelter gas scrubbing. These wastewater sources require neutralization of free acid in addition to soluble heavy metals removal. Both treatment requirements are conventionally accomplished in the same process by pH adjustment with an alkaline source to produce a wastewater pH optimum for precipitation of the metals present as hydroxides, carbonates, sulfates or other salts.
The removal of soluble metals by oxidation or reduction is utilized to obtain minimum solubility when precipitating iron and chromium. Iron in the ferric (Fe+³) form is less soluble than ferrous iron (Fe+²). Chromium, on the other hand, is chemically reduced from Cr+6 to Cr+³ before precipitation by pH adjustment with lime to precipitate chromium hydroxide.
The waste metals sludge that results from conventional lime neutralization of wastewater typically settles slowly in gravity clarification equipment and is very difficult to dewater by filtration. A small wastewater treatment plant process change has resulted in large benefits. Process development work conducted during the 60’s on treatment of acid mine drainage (AMD) from coal mines revealed that contact of lime used for neutralization and soluble metals precipitation, in this case iron, with recirculated sludge from the process clarifier prior to contact with the actual wastewater produced a condition which resulted in a highly settleable sludge easily dewatered by filtration.
The above has presented a brief overview of wastewater treatment practices currently used for heavy metal removal and other methods that will see possible use in the future. The results presented are for alkaline precipitation of actual mixed metals wastewaters treated at a pH of 9-9.5 units and after liquid-solids separation to produce an effluent suspended solids content of less than 5 mg/l.
Case Histories
A zinc concentrating and smelting facility produces 0.8 to 1.5 MGD of wastewater. Approximately 85% of the wastewater is discharged from the concentrating plant and the remainder comes from smelting air pollution control facilities. The process wastewater is conveyed first to an equalization lagoon having a liquid retention time of 5 to 6 days to remove a majority of the suspended solids present and to equalize the other wastewater characteristics prior to further treatment. The acid scrubber wastewater from the smelter air pollution control system flows directly to the wastewater treatment plant.
The treatment plant was originally operated with recirculation of thickened waste sludge to the neutralization tank to provide nuclei for the precipitation reaction and to prevent scaling of the process equipment. This practice has been discontinued because there was no demonstrated benefit of the sludge recycle to effluent quality from the Reactor-Clarifier treatment unit.
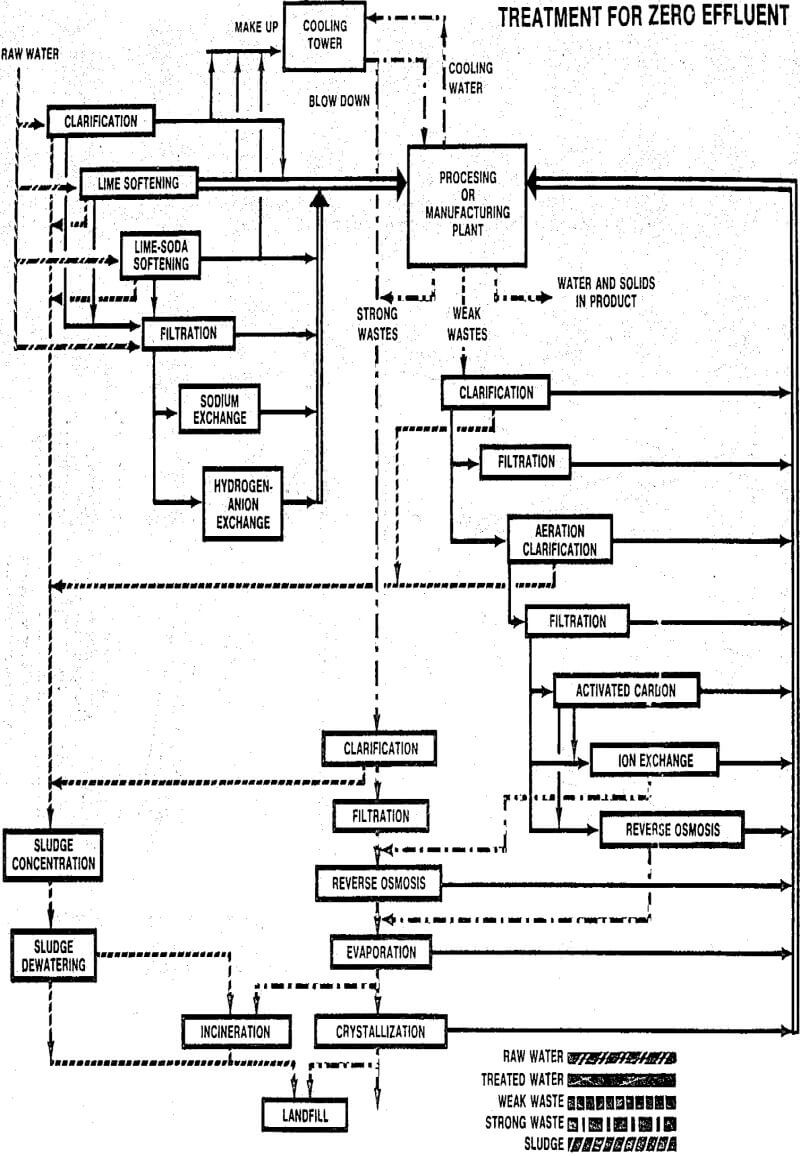
A zinc company operates an underground mine and processing plant which discharge approximately 6.5 MGD of wastewater. Over 60% of this flow comes from the mine operation and the remainder from ore processing. Figure 5 illustrates the flow sheet of the treatment plant used to treat the combined wastewater. The treatment process design is patterned after that originally developed for treating acid mine drainage from coal mines. In this process the lime used for wastewater neutralization and heavy metals precipitation is first contacted with a portion of recycled waste sludge.
The treated wastewater produced by the above plant is reused and also discharged to a river. The portion recycled for reuse is stored in a 2 MG lagoon to provide a source of water for fire protection. The water is then pumped to the zinc ore processing facility and reused in grinding and concentrating.