Table of Contents
Ranchers began its evaluation of the Bluebird Mine in late 1963. The property which included some 400 acres, adjoined one of the country’s leading producers – Inspiration Consolidated Copper Company. Such proximity led many to equate availability with undesirability. However, a relatively short period of exploration and metallurgical evaluation and a simultaneous assessment of other environmental elements led Ranchers to acquire the property and to expand its operation. The objective steady, continuous production, at a substantial level. The property was to become one of the first medium-sized copper mining operations to rely solely upon heap leaching as a means of production.
Development
Ranchers Exploration and Development Corporation obtained a 90-day option on the Bluebird property from Stovall Copper Company in January 1964. A 6,000 foot drilling program was initiated, and it was soon evident that the deposit consisted entirely of oxide ore. The economics of both vat and heap leaching were studied. Costs relating to mining, crushing, ore, waste and tailings handling, leaching, and recovery were examined closely. Leaching factors which received the most attention were acid consumption and costs and labor, power, and water costs. Since precipitation using iron was believed most feasible at the time, iron consumption and cost and labor, power and cement copper handling costs were the recovery aspects most carefully evaluated.
Production at this mine was to be dependent solely upon leaching – the first operation of its size with the specific objective of heap-leaching copper ore, not waste, tailings, or overburden. With this distinguishing factor in mind, it was obviously necessary to have as much control over the leaching solution as possible. Heap areas had to be selected which offered the best possible solution control and which would not interfere with the ultimate development of the mining plan. To avoid serious solution losses through faults in the leaching areas, it was decided to cover the entire area with soil cement. This, in effect, created a four-inch concrete pad composed of soil mixed with six percent cement, wetted and compacted.
To further control solution flowing to and from the leaching area, the cemented portion was divided into nine separate natural drainage sections, each containing its own perforated drainage pipe. Gravity would take the solution down through a heap, into its drain pipe, and downhill to a common collection point.
In Arizona where water is scarce, evaporation must be reduced whenever possible. Also, in an operation where one cannot dispose of water even if he wished to do so, its reuse is mandatory even though the water is immediately contaminated by the iron put into the solution at the precipitation plant.
Mining
In an operation which uses high cost methods to extract the copper value from the ore and recover it from solution, mining costs are particularly critical. Such is the case at the Bluebird Mine where ability to rip the ore and move it to the heaps at reasonable rates has been a key factor in making the mine a profitable operation.
Little difficulty was experienced in ripping the first 2-½ million tons of ore and overburden. A single shank was used on the parallelogram ripper which penetrated from 2 to 3 feet most of the time. This tractor kept enough material ripped to maintain a mining rate of 5 to 6,000 tons per day. The second ripper tractor was used for pioneer work, drill sites, and as standby for the primary ripper.
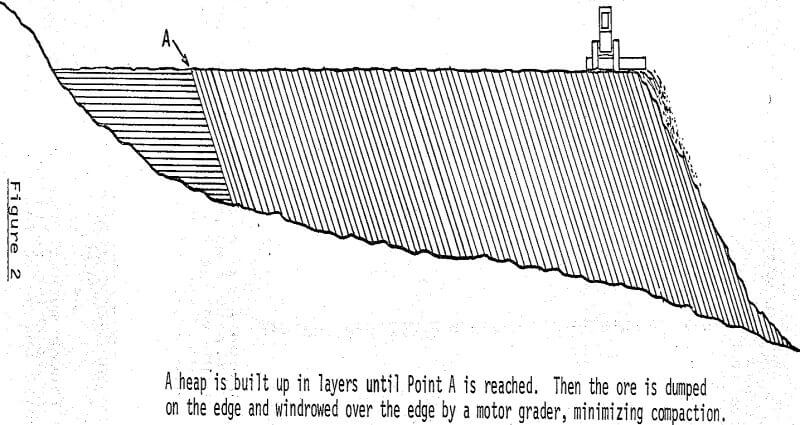
After the ore is ripped and push-loaded, the scrapers carry it about a quarter of a mile to one of the nine leaching areas. The ore is dumped, on the move, in the leaching area in such a manner that a roadway is built up to the elevation of the heap that is being built. During this building process, a motor grader keeps the heap surface relatively smooth to permit the scrapers to maintain their speed and to minimize tire damage.
Leaching
Ranchers began its leaching operation after several months of metallurgical testing, which included ore analysis, percolation and compaction tests, and evaluation and study of particle size, acid consumption, and recovery. Also evaluated were the effects of time, temperature, and acid strength, the behavior of the iron solution in the heaps, and the effect of iron on recovery and acid consumption. The data generated in these studies were useful not only in determining mining and heap construction methods, but helped to establish procedures for solution control, acid control, and leaching cycles.
The objective of the leaching cycle is the recovery or extraction of a majority of the copper content in the first leaching period. The remaining copper will be re-worked after a new lift of 18 to 20 feet of fresh ore is placed on top and leaching started again. The leaching cycle on a lift of fresh ore is to be 60 to 90 days – the exact cutoff to be determined according to the acid and copper in the off-solution. Acid application during the leaching cycle is keyed to the acid content of the off-solution – the higher the acid coming off, the lower the acid applied. The initial leach solution contains 50 grams per liter of sulfuric acid. This is gradually reduced over time to about 10 to 15 grams per liter near the end of the cycle.
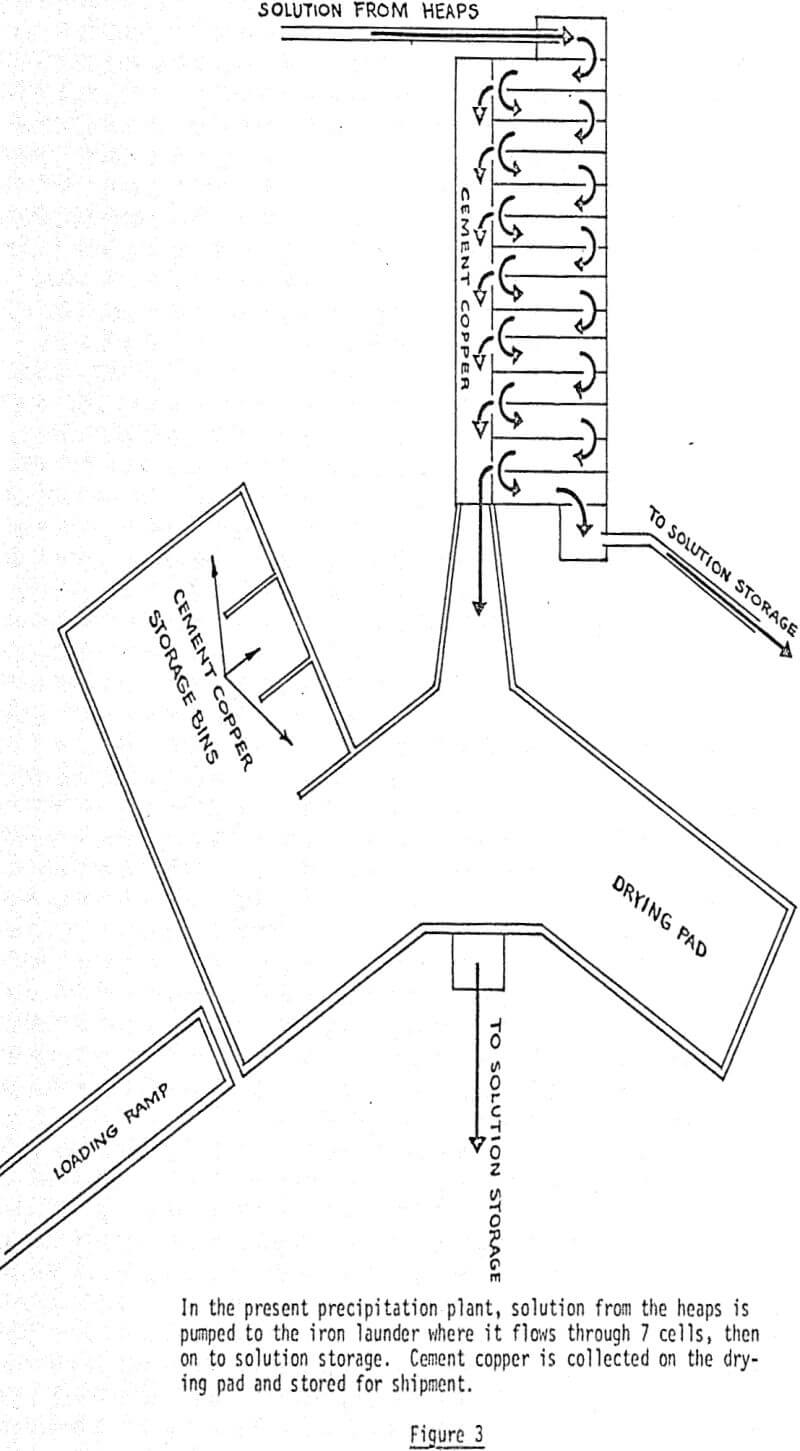
Precipitating copper from copper sulfate solution by means of iron is a process that has been used for centuries. The Bluebird Mine system differs only in that the solution going into the plant is relatively high grade, ranging from 3 to 10 grams per liter of copper. The precipitation plant, which was built by the prior owner of the property, was designed to receive 300 gallons per minute of solution and to produce 20,000 pounds of copper per day.
Solvent Extraction – Electrowinning
Since Bluebird ore contains relatively few impurities which are soluble in sulfuric acid solutions, and since the iron in the circuit would be eliminated when tin cans were no longer used for precipitation, it appeared that the off-solution from the heaps would be a fairly pure copper sulfate, ideal feed for solvent extraction. The typical off-solution from the heaps is high in acid, which affects the efficiency of the solvent extraction step, and this caused some concern.
The decision whether to build or not had to be based on the outlook for the electrolytic copper price for the next several years. The long range outlook for the marketability of cement copper also had to be considered. The Bluebird was able to take advantage of the premium price for cement copper in 1964 and 1965, and in early 1966, it continued to appear that premium sales of cement copper could probably be made for the remainder of 1966 and probably all of 1967. So, despite favorable pilot plant results, a decision was made to continue production of cement copper for the time being.
The basic element involved in solvent extraction is an organic reagent which is unusually selective in extracting copper from leach liquor by a process called liquid ion exchange. A 7 to 10% solution of this reagent in a carrier such as kerosene surrenders two hydrogen ions in exchange for each copper ion in the leach liquor. The barren leach liquor is then returned for leaching. In a second ion exchange process, the copper is stripped from the organic reagent by an acidic electrolyte in exchange for hydrogen ions. This extraction and stripping takes place in a series of mixers and large settling basins. Following this extraction and stripping, the copper is plated out of the electrolyte in an electrolytic cell in which electric current passes from lead anodes to thin copper cathode starting sheets. Copper is deposited on the starting sheet to a thickness of about ¾ inch and is more than 99.9 percent pure.