Table of Contents
An estimated 17 million mt of manganese are contained in domestic manganese-bearing silicate resources, yet domestic production is insignificant compared with the quantity of manganese imported yearly into this country for consumption. The projected U.S. consumption of manganese in 1990 was expected to be about 680,400 mt with the majority of total demand being supplied by foreign sources. Continuing dependence on foreign manganese ores will persist unless domestic resources are utilized.
The manganese silicate ores of the Silverton Mining District of San Juan County, CO, have been estimated to contain 2.4 million mt of manganese. These extensive manganese deposits have received little consideration as a source of manganese because promising processes for converting manganese in a silicate mineral into more usable forms have not been developed. Previous investigators have proposed a combination pyrometallurgical and hydrometallurgical process to treat the manganese silicate ores. The process consisted of arc melting the ore at 1,550° C and then rapidly cooling the melt by quenching it in water.
The resulting amorphous product was acid leached for manganese extraction.
Becausc of their crystalline structure, silicate minerals are not readily attacked by a mineral acid unless the crystalline structure is altered. Research conducted on aluminum silicates demonstrated that HCl leaching systems incorporating low levels of a fluoride source were successful in leaching Al from kaolinite and anorthosite. Acid, with small amounts of fluoride ion, disrupts the silicate structure sufficiently to enable effective leaching.
The U.S. Bureau of Mines has been conducting bench-scale studies to determine the feasibility of leaching a Mn- bearing silicate ore with acid and fluoride as an alternative to extractive techniques requiring a high-temperature and energy-intensive pretreatment step. Droes described the effect of CaF2 on sulfuric acid (H2SO4) leaching of a Mn-bearing silicate ore. This report presents the results of CaF2-enhanced HCl leaching of a manganese silicate ore.
Procedures and Equipment
The ore used in this study was from the Sunnyside Gold Corp., located in the Silverton Mining District of San Juan County, CO. The elements of primary interest contained in the ore were, in percent, 35.1 Mn, 23.4 Si, 1.8 Ca, and 1.4 Fe. Ore characterization and mineralogical examination were accomplished using microscopic, X-ray diffraction, and X-ray fluorescence analytical techniques. Major minerals present were rhodonite (MnSiO3), pyroxmangite [(Mn,Fe)SiO3], with trace amounts of quartz (SiO2), and rhodochrosite (MnCO3). A nominal ore size of approximately 80 mesh (0.007 in) was used in this investigation. The HCl and CaF2 used were reagent grade.
Leaching tests were performed in a 0.5-L glass kettle fitted with a ground glass cover. A charge consisting of 53.8 g of ore was mixed with 100 mL of water within the reaction kettle. The solids were mixed with a Teflon fluorcarbon polymer paddle-type stirrer rotated at 750 rpm. The kettle vent was attached to a water-cooled condenser to reflux and return acid to the kettle. Heat was supplied by an electric heating mantle, which was controlled with a thermocouple temperature controller.
When the desired reaction temperature was reached, measured quantities of HCl and CaF2 were added through a cover hole. After leaching, the slurry was vacuum filtered through Whatman No. 5 filter paper on a Buchner funnel. The leached residue was displacement washed once with water, filtered, and dried at 90° C for 24 h.
Manganese was recovered from solution as MnCO3. Reagent-grade sodium carbonate (Na2CO3) was added to raise the pH to approximately 8.0 followed by 2 h of stirring at 50° C. The MnCO3 precipitate was filtered from solution, washed, and dried. Qualitative and quantitative analyses of the MnCO3 product were performed to determine its quality and purity.
The EPA EP test was conducted on selected solids. The procedure consisted of pulping 1 part solids with 16 parts deionized water, adjusting the slurry pH to 5 with acetic acid (if necessary), agitating for 24 h, and filtering. The filtrate was analyzed for the toxic constituents of concern.
Metal values in the solutions and solids were analyzed by inductively coupled plasma spectroscopy. Wet chemical analysis determined free acid, Cl-, and F- species. A combination of X-ray diffraction and X-ray fluorescence analyses provided compound identifications and characterizations.
Results and Discussion
HCl-CaF2 Leaching
A preliminary experiment employing HCl and CaF2 to leach the manganese silicate ore indicated that extraction of Mn was possible. Approximately 90 pct of the Mn was extracted when a 9-pct ore slurry was leached with a 2.0M HCl-0.25M CaF2 solution at 50° C for 1 h. All subsequent experiments were conducted using a slurry density of 35 pct. This was done since a practical leaching operation would be conducted at the maximum percent solids proportionate with slurry transport, agitation, and minimum equipment size. Experiments were conducted to determine the leaching parameters for best extraction of Mn during CaF2-enhanced HCl leaching of the manganese silicate ore. These tests investigated the effects of the amount of HCl and CaF2 added during leaching, reaction temperature, and retention time.
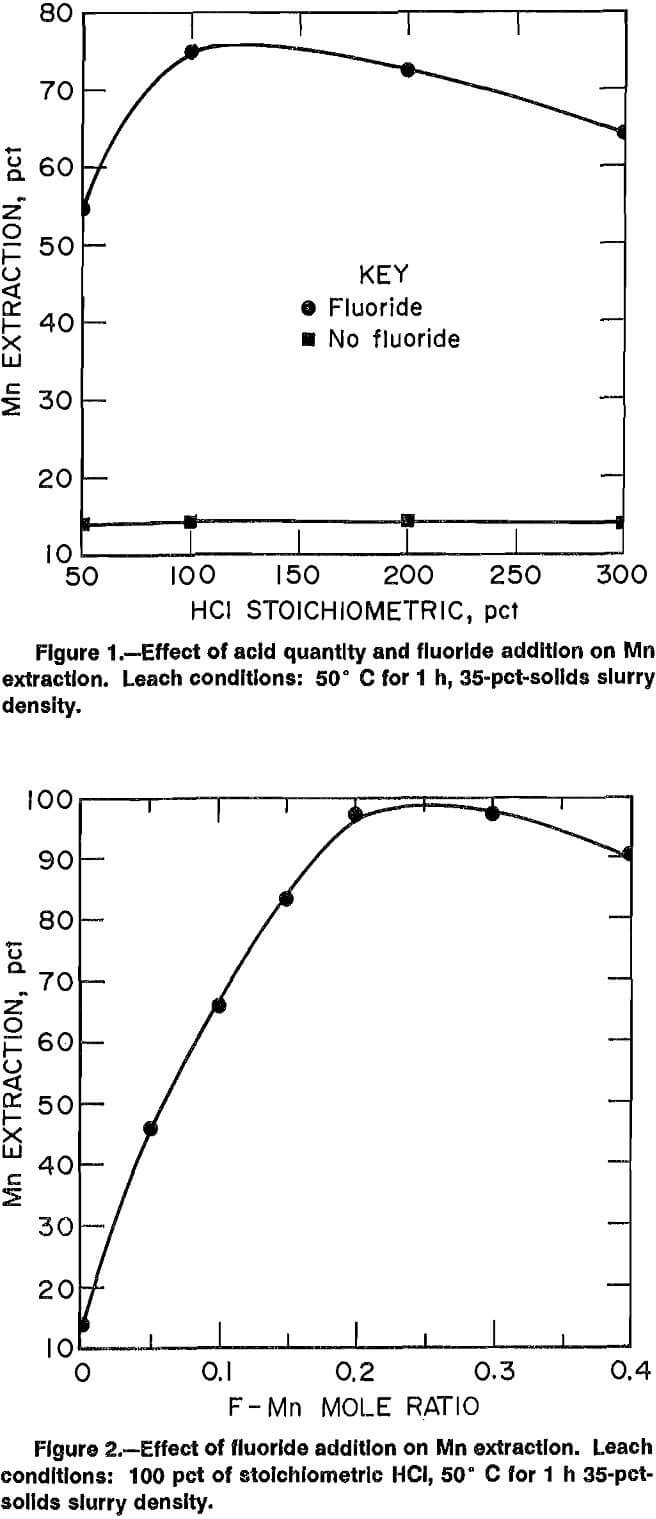
The quantity of HCl added during leaching was investigated to determine the effect on Mn extraction. Acid additions (50,100, 200, and 300 pct of stoichiometric) were tested based on the following simplified reaction,
MnSiO3 + 2HCl + H2O → MnCl2 + Si(OH)4…………………………………………………………….(A)
The theoretical HCl requirement (100 pct of stoichiometric) would be 0.56 g HCl per gram of MnSiO3 leached. Tests were made with and without the addition of F. The results are summarized in figure 1. CaF2 was added so that the ratio of moles F in the leach solution to moles Mn in the ore was 0.145. A 35-pct slurry density and a temperature of 50° C were used in all tests. Duration of each leach was 1 h.
If leaching was conducted with just acid, then only 14 pct of the Mn was extracted at the acid ratios investigated. The best Mn extraction during F-assisted leaching resulted when HCl was added at 100 pct of stoichiometric. Providing an excess of acid during HCl-CaF2 leaching did not increase Mn extraction.
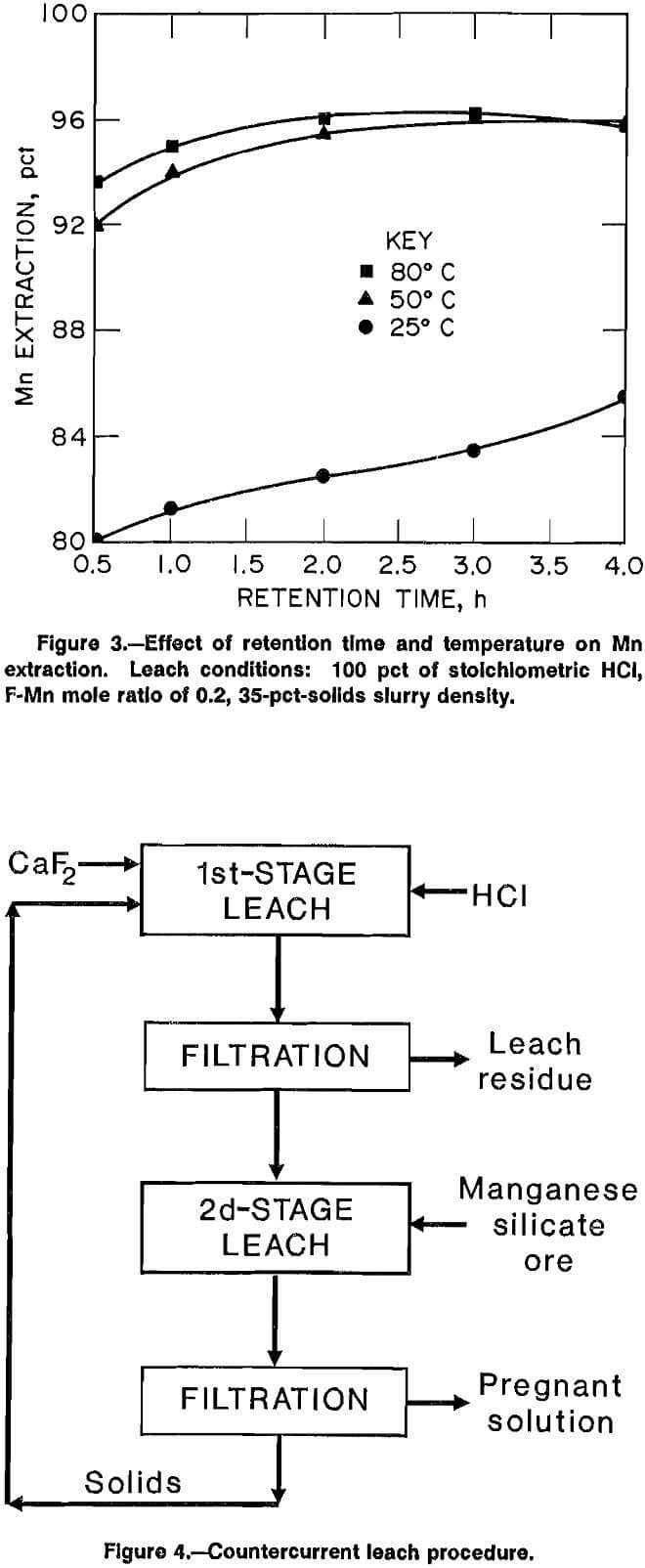
Experiments to determine the effect of F addition on Mn extraction were conducted by leaching a 35-pct ore slurry with a constant quantity of HCl (100 pct of stoichiometric) and variable amounts of CaF2. All tests were performed at 50° C for 1 h. CaF2 was added so that the mole ratio of F in solution to Mn in the ore was at 0.05, 0.1, 0.15, 0.2, 0.3, and 0.4. Test results are summarized in figure 2. Addition of CaF2 to the leach solution to provide a F-Mn mole ratio of 0.2 resulted in 96 pct Mn extraction compared to 14 pct Mn extraction when no CaF2 was added. Further F additions (F-Mn >0.2) to the leach solution did not increase Mn extraction, but did increase the solubilization of Si from the ore. Aqueous concentration of monosilicic
acid [Si(OH)4] increased from 1.4 g/L at a F-Mn ratio of 0.2 to 7.5 g/L at a F-Mn ratio of 0.4. The high Si(OH)4 concentration in solution would be subject to silica gel formation in subsequent process steps and therefore, a F-Mn mole ratio of 0.2 was used in future tests.
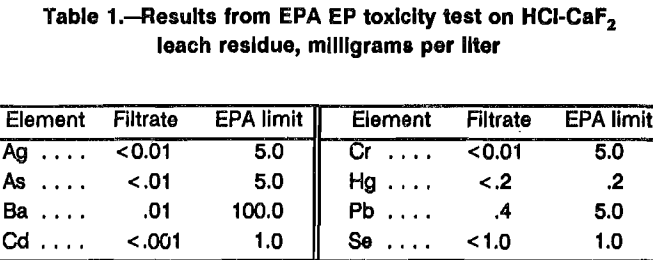
Manganese extraction was evaluated as a function of reaction time and temperature. The experiments consisted of placing 53.8 g of ore and 100 mL of water in the reactor and heating to the desired operating temperature. HCl and CaF2 were then added to provide 100 pct of stoichiometric HCl (0.56 g HCl per gram of ore) and 0.20 mole F in solution per mole Mn in the ore. In these tests, the temperature was maintained within ±2° C of the desired values. Samples for Mn analysis were taken after 0.5, 1.0, 2.0, 3.0, and 4.0 h at temperatures of 25°, 50°, and 80° C. Each residue was thoroughly washed to remove soluble Mn and analyzed. Experimental results are shown in figure 3. Elevated temperatures were required for the highest Mn extraction. Although Mn extractions were slightly higher in the 80° C test at reaction times <3 h, 96 pct of the Mn was extracted in the 50° C test when the reaction time was >3 h.
Results from these experiments indicated that leaching a 35-pct manganese silicate ore slurry at 50° C with 100 pct of stoichiometric HCl (0.56 g HCl per gram of ore) and a F-Mn mole ratio of 0.2 for 3 h would extract 96 pct of the Mn. Approximately 83 pct of the fluoride added remained in the leach solution, while 17 pct reported to the leach residue. The leach solution produced under the above conditions contained, in grams per liter, 168 Cl-, 87.8 Mn, 9.1 Ca, 5.53 HF, 2.7 Fe, and 0.67 Si.
Countercurrent Leaching
Countercurrent leaching was conducted to utilize excess leach acid and improve Mn extractions and to recover and recycle the fluoride that had been added to enhance leaching. The manganese silicate ore was leached countercurrently in a procedure schematically illustrated in figure 4. In the first stage, a 35-pct-solids slurry of ore leached in stage 2 was leached at 50° C for 3 h with a fluoride-bearing leaching medium containing 100 pct of stoichiometric HCl (0.56 g HCl per gram of ore) and F-Mn mole ratio of 0.2. Leach solution from the first stage was sent to the second stage and contacted with fresh ore. Ore was added to produce a 35-pct slurry density. After allowing the slurry to mix for 5 h at 50° C, it was filtered and the concentrated pregnant solution was subsequently treated for Mn recovery. The solids from the
second stage were advanced to the first stage and the procedure was repeated. These solids normally contained, in percent, 32 Mn, 20.5 Si, 1.4 Ca, 1.02 F- and 0.94 Fe. Water and acid were added in the first-stage leach to make a 35-pct-solids slurry and to provide 100 pct of stoichiometric HCl, respectively. Replacement CaF2 was added so that the F-Mn mole ratio was 0.2. Leaching was conducted at the conditions previously defined.
A series of three two-stage leach tests were performed to simulate a continuous countercurrent leach process. In the initial test, a simulated leach solution was prepared that would be representative of a first-stage leach. When this solution was treated in the second stage, as shown in figure 4, 17 pct of the Mn was extracted from the ore added. Advancing the ore from this stage to the first stage and adding fresh acid and makeup CaF2 solubilized 99 pct of the remaining 83 pct of the Mn contained in the ore. Between the two leaches, a combined extraction of 99 pct Mn resulted. The other two tests were conducted in a similar fashion using the preceding test’s first-stage leach solution. Combined extractions of 98 pct Mn resulted as steady-state conditions were established.
Countercurrent leaching improved Mn extraction compared with single-stage leaching using a corresponding amount of acid and fluoride. Excess leach acid from the first stage was utilized in the second-stage leach. The recycled solids and solutions did not affect leaching. Total Mn extractions of 98 pct resulted. As excess acid was consumed and the slurry pH was increased, fluoride in solution precipitated. In each test, 99 pct of the fluoride in the leach solution from the first stage was recovered and recycled. Although most of the fluoride dissolved during the leach was recovered, a makeup of about 20 pct was required in the next countercurrent leach cycle to account for the fluoride lost in the leach residue.
An EPA EP toxicity test was conducted on the HCl-CaF2 leach residue to determine if it could be classified as nonhazardous. The leach residue was primarily SiO2 as identified by powder X-ray diffraction and contained, in percent, 32 Si, 9 Mn, 1.1 F, 0.3 Ca, and 0.3 Fe. Results of the EPA EP toxicity test are shown in table 1. Disposal of the residue should not impact the environment since concentrations of metal values in the extract from the test were less than the maximum allowable concentrations of toxic constituents established by EPA for a nonhazardous waste. The extract also contained 70 ppm Cl- and 3.3 ppm F-. Owing to the concentrations of the chloride and fluoride anions in the extract, steps may have to be taken to meet site-specific effluent discharge requirements.
Manganese Recovery
The pregnant solution from the countercurrent leach procedure was rich in Mn. A typical pregnant solution assay was, in grams per liter, 150 Cl-, 111 Mn, 7.63 Ca, 3.5 Fe, 0.047 Si, 0.02 HF, and the pH was about 4.5. Manganese carbonate (MnCO3) was precipitated by the following reaction,
MnCl2(aq) + Na2CO3(s) → MnCO3(s) + 2NaCl(aq)……………………………………….(B)
Essentially complete precipitation of the Mn as MnCO3 resulted when Na2CO3 was added to increase the pH to 8.0 followed by 2 h of mixing at 50° C. Greater than 99 pct of the Mn was precipitated using only 75 pct of the stoichiometric Na2CO3 requirement. The Mn precipitate was approximately 85 pct MnCO3 by weight.
Powder X-ray diffraction analysis of the precipitate indicated that MnCO3 was the only crystalline phase present in the solids, but chemical analysis of the solution and the solids indicated some coprecipitation of impurities. It is suspected that the Ca may have replaced some of the Mn in solid solution. After Mn recovery, concentrations of species in solution were, in grams per liter, 50 Cl-, 30 Na, 2.2 HCO3-, and 0.0012 Mn. In practice, the effluent from the Mn recovery step would be recycled and used as process water.
Process Flowsheet
The proposed flowsheet to recover Mn from a domestic manganese silicate ore is shown in figure 5. The ore is countercurrently leached with HCl and CaF2, at 50° C to solubilize the Mn. In the second stage of the countercurrent leach procedure, excess leach acid is utilized to produce a concentrated Mn solution and most of the fluoride reagent is recovered and subsequently recycled. The final leach residue (from the first stage-leach) is classified as a nonhazardous waste.
The addition of Na2CO3 to the pregnant solution precipitates MnCO3, which could be further refined and sintered to produce metallurgical-grade manganous-manganic oxide (Mn3O4) and should be suitable as a feed for ferromanganese-producing furnaces. The MnCO3 precipitate would also be a suitable feed for an electrolytic Mn operation and could be used as a raw material for making other Mn chemicals. The flowsheet shows the effluent from the Mn recovery step with all wash solutions being recycled and used as process water in the leaching operation. These solutions could also possibly be used as wash water in the filtration steps. In practice, it would be necessary to include an additional step in the flowsheet to alleviate the buildup of sodium chloride (NaCl) in the process solution. NaCl could be crystallized from solution by HCl gas sparging. The evaluation and optimization of this step were beyond the scope of this investigation.

Summary and Conclusions
This investigation has demonstrated that CaF2-enhanced HCl leaching was effective in extracting Mn directly from a domestic manganese silicate resource. Countercurrent leaching resulted in a more efficient use of acid and fluoride than single-stage leaching. An overall Mn recovery of 98 pct resulted when the ore containing 35 pct Mn was processed according to the proposed flowsheet. Unit operations in the process flowsheet had low temperature requirements. The leach residue produced can be classified as nonhazardous according to results of the EPA EP toxicity test. Since Mn was successfully extracted directly from the ore without a high temperature and an energy-intensive roasting step, the proposed hydrometallurgical process has potential for decreasing energy consumption for processing domestic manganese silicate resources.
