Handheld XRF spectrometers are a fast, accurate, and portable tool that can be used for a variety of tasks in the mining industry.
What is a portable spectrometer and how do you use one?
A handheld “XRF gun” is an x-ray instrument used for chemical analyses. Shaped and sized similarly to a barcode scanner, the device is held in one hand, and typically weighs about 1.2 kg (~0.5 lb).
 |
 |
Chemical analyses are conducted by placing the sample window (the front of the device) in contact with the sample or as close to the sample as possible to minimize radiation backscatter.
Analysis is initiated by pressing the trigger finger button, which fires x-rays into the sample. X-rays characteristic of composition are released from the sample and counted by the detector on the front of the device. The time required for analysis depends on the nature of the samples and the elements analyzed. Major element analyses typically take a few seconds, whereas minor to trace element analyses typically take a few minutes. Following analysis, the test results are stored and then reported on a digital interface located on the top of the instrument.

What sample types are compatible for a handheld XRF? Which elements can be detected?
Typical samples include but are not limited to rocks, minerals, sediments, and fluids. Current devices can detect elements as low as Mg (atomic number 12) and as high as uranium (atomic number 92).
Older devices detect less elements and have higher detections limits. Visit our XRF Discussion Forum
Calibration standards what are they and do I need them?
Calibration standards are samples with known compositions that are used for instrument calibration and quality control. Although they are not required for analysis they are highly recommended for scientific and industrial applications to ensure that the device is operating correctly.
How does x-ray fluorescence work?
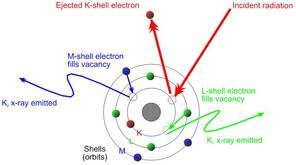
X-ray fluorescence is a process in which electrons are displaced, resulting in a release of energy characteristic of a specific element. A more detailed breakdown of the process is listed below:
- X-rays are generated by an x-ray tube.
- The X-rays exit the x-ray tube, where they enter and interact with the sample.
- The X-rays displace only those electrons that have less binding energy than the energy of the X-ray beam.
- The displaced electrons from lower orbits (lower energy states) make the atom unstable and are filled by electrons from higher orbits (higher energy state), releasing energy in the form of X-rays; which are characteristic of an atom due to the unique spacing between the orbital shells of each atom.
5. The X-rays emitted from the atoms are counted by the detector located at the front of the XRF device and used to determine the quantity of each element present.
Who makes handheld XRF analyzers?
There are many companies that manufacture handheld XRF Spectrometers including:
- Bruker
- Thermo Scientific
- Olympus
- SPECTRO
XRF Sample Preparation Pellet Press
Image 1 Source
Image 2 Source
https://commons.wikimedia.org/wiki/File:Analysis_of_tablets_by_portable_XRF.jpg
Image 3 Source
https://www.olympus-ims.com/es/applications/handheld-pmi/
Image 4 Source
https://www.bruker.com/fileadmin/user_upload/1-Products/X-rayDiffraction_ElementalAnalysis/HH-XRF/Misc/Characteristic_X-ray_production1.jpg
This is a guest post written by GeoMetallurgist Joshua Wright.