The general use of chemical grinding aids involving several different mechanisms for increasing throughput in the wet grinding of ores has been discussed in a number of recent publications. It appears from industrial scale testing that the use of selective dispersants with certain prespecified operating conditions is an approach which can produce two important results: first, increased feed rate at constant product size and second, the production of a finer product at constant feed rate. The engineering mechanism involved with the use of selective dispersants is one of allowing more dense slurries to interact with the tumbling media while still maintaining the slurry fluidity and mass transport characteristics of less dense slurries.
A number of publications contain examples of laboratory and industrial scale usage of an experimental grinding product developed. This product, denoted as XFS-4272.00 is a low molecular weight water soluble anionic polymer which operates as a selective dispersant. It has demonstrated significant industrial results in a number of trials if certain operating conditions exist and the product demonstrates certain performance characteristics in the grinding system under study. Readers interested in industrial results should consult the above references for further information.
The purpose of this paper is to demonstrate the laboratory evaluation of XFS-4272.00 on a particular ore in a rather complete manner with both a standard laboratory batch ball mill and a small open circuit continuous laboratory ball mill. Such a sample demonstration is important as it is on such laboratory mills that most industrial feasibility studies are usually performed. The laboratory scale results have been shown in numerous evaluations to be scaleable to industrial mills with a common set of principles underlying the entire process regardless of the size of the mill or mode of operation (e.g. batch on continuous).
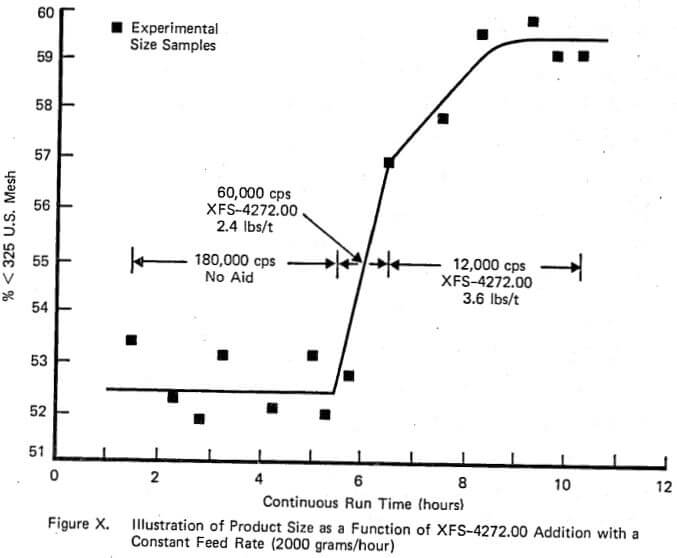
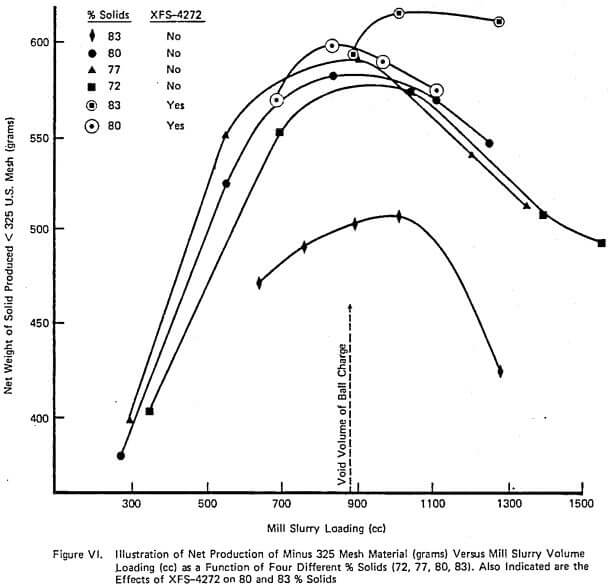
As mentioned earlier, the purpose of running a few experiments in a small continuous mill was to demonstrate the similarity of behavior between large and small scale continuous mills. Figure X illustrates very well the variation in weight % of product passing 325 mesh at a constant feed rate of 2000 grams/hour versus time under three different operating conditions: standard operation without chemical, 2.4 lbs/t of XFS-4272.00, and 3.6 lbs/t of XFS-4272.00. The increase in % passing 325 mesh (7%) plus the reduction in viscosity is obvious. This is exactly the same pattern that is observed in large over-flow discharge industrial mills except that typically a dosage rate of 0.6 lbs/ton would give an increase of 4-5% passing 325 mesh.
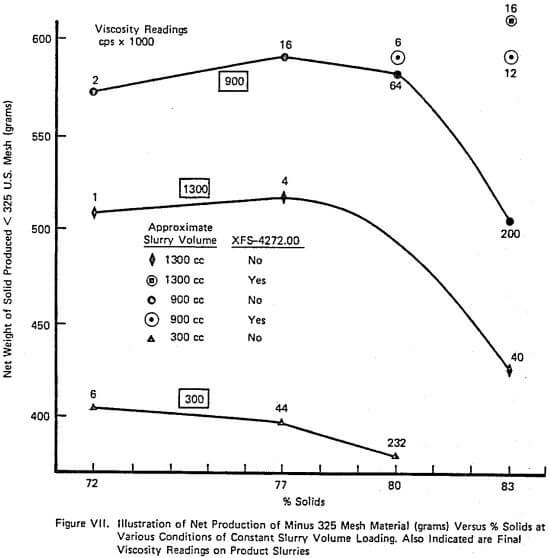
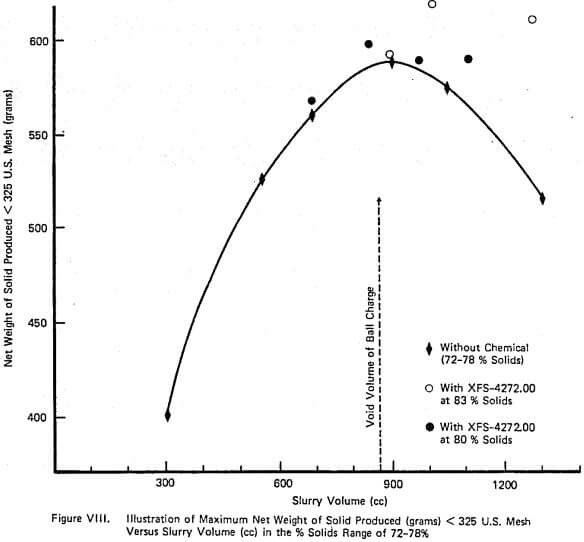
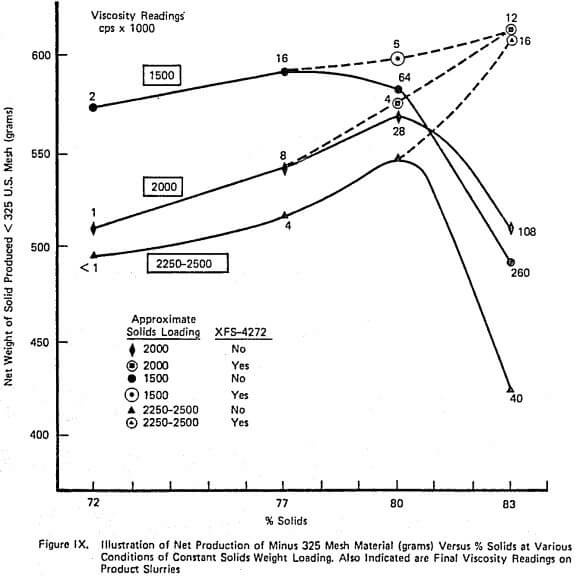
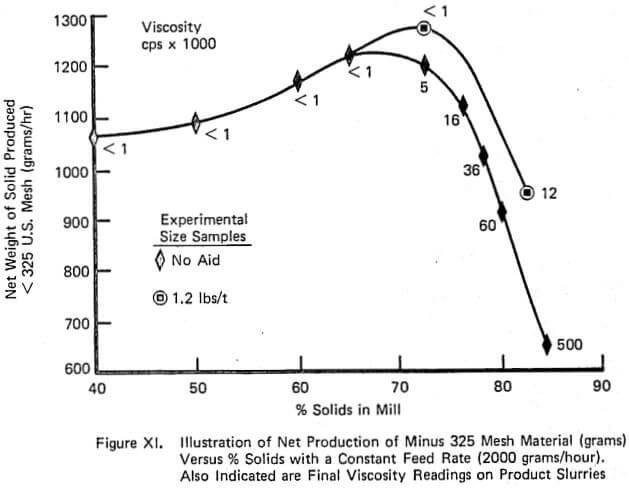
Figure XI demonstrates the continuous counterpart of the type of information contained in Figure VII and again duplicates industrial data well. The relationship between net production and % solids is relatively flat over a large range of % solids until viscosity becomes sufficiently large. The addition of grinding aid increases the net production that can be achieved. Also, the shape of the net production curve with and without aid is similar which is also observed on large scale continuous mills.
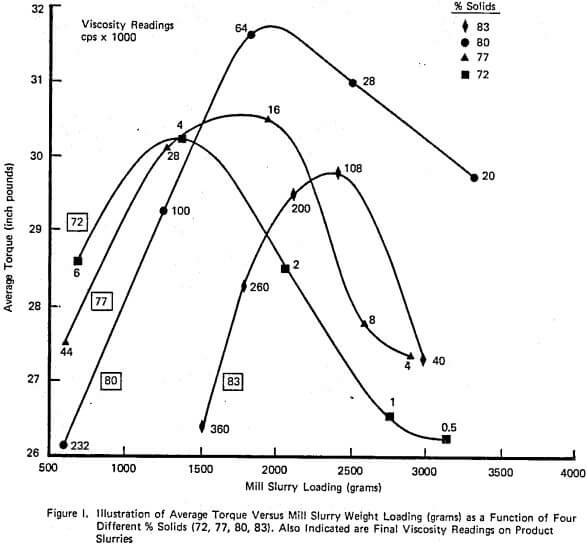
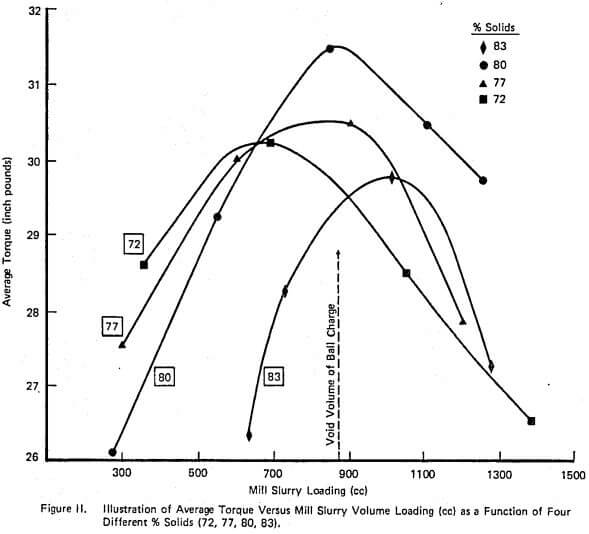
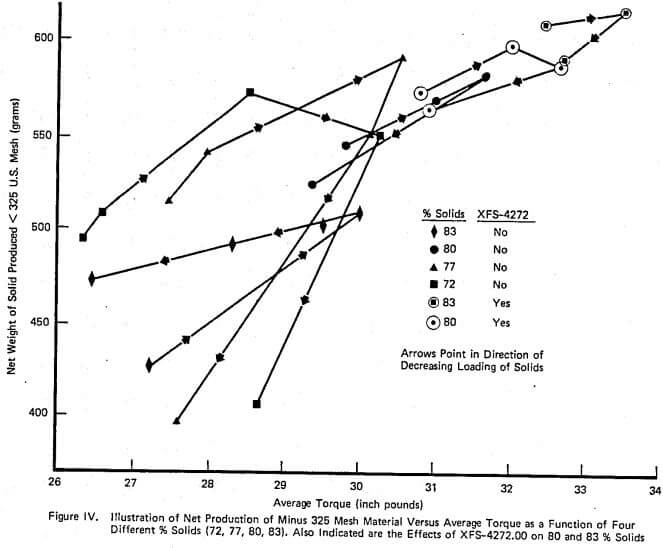
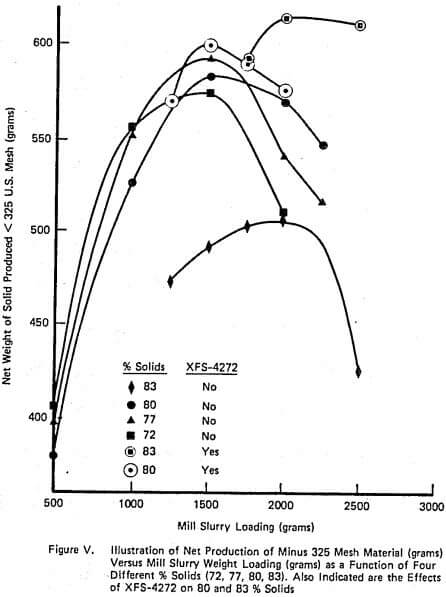
A complete laboratory scale characterization of the effect of selective dispersants as wet grinding aids on a given ore was illustrated using XFS-4272.00. A variety of data sets were collected to show that consistent correlations involving torque, viscosity, % solids, net production rate, and slurry weight and volume loading exist both with and without chemical.
The relationship between such laboratory data and industrial scale testing results has also been shown to be very consistent. Successful use of the selective dispersant approach requires a consistent chemical behavior and proper adjustment of operating conditions within the mill.
The effect of using the dispersant approach, e.g. XFS-4272.00, to improve grinding can be significant with some continuous industrial tests giving 8-15% increases in throughput of similar product size or a 3—6% finer grind at 200 mesh at constant feed. The data presented also demonstrate that the effective use of selective dispersants requires that a mill be in a situation where significant viscosity effects would be present without the chemical. This condition usually corresponds to a set of operating parameters where normally the mill would be demonstrating a decrease in net throughput without chemical. For an operation which gives a constant slurry volume, the dispersant effect is very easy to quantify by merely changing the % solids. Thus in a continuous mill with chemical, for example, one has the option of holding feed water relatively constant and increasing the feed or keeping the solids feed rate constant and lowering the amount of feed water. The operating characteristics of using the dispersant approach are very similar to normal water control as there is no change in the relative manner in which particles are broken only a change in the absolute rate.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
GRINDING ADDITIVES
The term “additive” is applied, in milling practice, to various substances which, when added to the contents of a mill, bring about an improvement in the rate of grinding and, therefore, a reduction in the time of grinding required to attain a specified degree of fineness. Additives could also be introduced in order to attain some special characteristic of the final product. However, such applications are dependent on the purpose of the final product and so an outside the scope of the present chapter. An interesting feature of additives is that the quantity required is generally very small; usually being between about 0.01% to 0.1 % of the weight of the charge treated.
That additives are of sufficient importance industrially to warrant consideration is shown by recent work of Skaupy, in which it is shown that the rate of milling of iron powder in iso-amyl alcohol is no less than twenty times the rate in dry air. These tests were carried out on a vibration mill, and so might not be directly comparable with a ball mill, but nevertheless the result is significant. In view of the large energy requirements for grinding, especially fine grinding, a saving of even 20% in milling time could make a substantial saving in milling costs.
In view of the potential importance of these substances it is perhaps surprising that their mode of action is not precisely known. It is impossible to establish from the literature on the subject whether their action arises from improvement in the conditions of crushing by the prevention of a coating of powder on the ball surface, from the prevention of agglomeration in dry milling, or flocculation in the case of wet milling, or by some action within the particle, such as the promotion of the spread of the fissures which occur naturally in all solid materials. It is possible, indeed probable, that all these modes of operation are of importance, either singly or in various combinations, depending on the circumstances and the controlling conditions present.
For dry milling, the additive may be present in solid form, in the form of a solution added to the dry feed, or in a gaseous or vapour state. For wet milling, the additive is present in the water; the concentration generally being less than 1% of the solid material present.
Before discussing in detail the use of additives in milling it is perhaps relevant to recall what has been said earlier. As shown in Chapter 4, for a substance in which the yield dress and the stress for fracture coincide, the strain energy eventually reappears as heat and the energy associated with the creation of new surface represents a very small fraction of the total energy involved in the crushing operation; this energy being only about 5% of the total when a particle is comminuted in a simple impact crusher. For very fine powders, however, the surface energy becomes more important and manifests itself in high adsorbtivity and the tendency to agglomeration observed in finely divided substances. Even so, the energy associated with the surface is a small proportion of the total involved. Because of this it is difficult to see how the reduction of the surface energy can significantly affect the energy required for grinding but, nevertheless, surface active dispersing agents are often very effective grinding aids. The reduction of the surface energy of the particle itself is not, however, the only possible explanation of the action of these materials.
A possible direct mechanism for the operation of these substances has been suggested by Rehbinder. Assuming that the molecules of the additive are adsorbed on the surface of the particles, then the cohesive forces which bond the surface centres of the crystal lattices, at the surface of the particle, will, in accordance with Gibbs’ equation, be lowered by an amount necessary for the adsorption to take place. Thus, the microscopic cracks and fissures, which are always present in brittle materials, will, after the adsorption process, be more easily widened. From this hypothesis two tentative conclusions, regarding the effect of such agents on the crushing process, may be drawn.
Firstly, when the particles are very small the physical strength would be expected to be affected. Also, with larger particles, the conditions existing at the crack tips are modified and this could lead to higher stress concentrations in these regions: these higher stress concentrations leading to reduced physical strength of the particle. Thus, the strength of particles of all sizes would be expected to be reduced but, on the whole, the finer particles would be expected to be most affected.
Secondly, the dispersive effect of the additive would be most marked with the small sizes of particle and so agglomeration would be reduced. This would lower the so-called “limit of fine grinding”, observed by mill operators, at which equilibrium is established between the rate of production of fine material and the rate of production of larger particles resulting from agglomeration and, possibly, from recrystallization, of smaller ones. An incidental effect would be that cohesion between the ball and layers of particles surrounding it would be reduced and that this would, by reduction of the thickness of the layers, reduce “cushioning” of the blow, with consequent loss of energy in the form of heat.
Strong evidence supporting this hypothesis is given by the work of Baker and Preston, on the tensile strength of glass. Experiments show that the tensile strength of glass is 20% greater when the specimen is dried in an oven than when the surface is wet and is as much as 200% greater when baked in a vacuum than in the wet condition. The tests also show that moisture is the chief factor in the loss of strength; although the presence of carbon dioxide also has some effect. Thus the surface condition of the glass affects the average tensile strength at failure, even for the case of rods of comparatively large diameter. The surface of glass is, in fact, always covered with microcracks, and the hypothesis put forward was that the water molecules are active at the crack tips and, that there is a local chemical attack, with the formation of a gel, which weakens the structure at this point. However, this may be, the tests demonstrate that the surface conditions of even large particles influence their strength in a decisive manner.
It is clear that, irrespective of the question of additives as such, the nature of the fluid medium in which the crushing process takes place would be expected to influence the energy required to produce a specified fineness. It is commonly stated that, all other things being equal, the energy requirements for wet grinding are as much as 20% less than for dry grinding. Practical experience also suggests that the limit of fine grinding is lower in wet grinding than in dry. This observation is in general accord with the principles discussed above.
The general picture which tends to emerge concerning the operation of additives is therefore that this is primarily in reducing the effective energy at the particle-fluid medium interface, due to the adsorption of the additive on the particle surface. This could result in decreasing the strength of the larger particles, but a much more pronounced effect would be expected in the very fine size ranges, where the surface of the particle becomes very much more important in relation to its volume. Also in the very fine size ranges, the reduction in the agglomeration tendencies among the particles would be expected to have a beneficial effect in reducing the energy for grinding and in reducing the grinding time. It can also be deduced that, in accordance with the above general principles, the most effective additives would be expected to be surface active substances.
Such considerations do not, however, explain the operation of solid additives in dry grinding; for example the improved mill performance obtained when a small quantity of carbon black is added to the cement clinker under going grinding. Here again, it is suggested that the function of the additive is in the prevention of cushioning since Schweitzer and Craig have observed that addition of carbon black in proportions as low as 0.08% is highly effective as a cleaning agent for the surfaces of the balls and mill.
A rather different explanation of the operation of additives is put forward by Bond; it again being suggested that ball coating is an important factor. These tests showed, in the first place, that with a soft material, such as limestone, excessive ball coating occurs with powders of much larger particle size than for hard materials, such as quartz. Furthermore the occurrence of coating is practically independent of the character of the grinding media , cast, forged and ball-bearing steel balls showing no significant difference other than that, when new, the smooth hard balls are not subject to coating. Once abrasion has removed the surface, however, these balls show the same behaviour as the others.
According to Bond, the ball coating consists of an innermost layer of particles, of about 0.5—50µ in diameter, wedged into the surface of the ball. The outer layers of particles making up the coating are keyed to this inner layer and are of much larger size. The effect of these layers is to cushion the grinding surface and so to greatly decrease the grinding rate. It is possible that other mechanisms are operative; for example the beneficial results obtained by the use of small quantities of carbon black as a grinding aid with cement clinker suggests some analogy with electrostatic precipitation. In this application it is known that the addition of a small amount of carbon, generally in the form of unburnt fuel, can bring about the precipitation of a flue dust which otherwise cannot be treated; the function of the carbon apparently being to form a conducting network through the layer of powder by which electrical charges leak away to the collector electrode. Is it not possible, therefore, that some such mechanism is present in this application?
The question of additives will now be considered in more detail; commencing with dry grinding. As previously stated, in dry grinding the additive may be present, at least in theory, in solid, liquid, vapour or gaseous form and some of the most interesting results of the use of these additives are reported from the cement industry.
According to Berry, advantages have been shown by the use of vinsol resin, cod oil, beef tallow, aluminium stearate and glycerol in the grinding of cement clinker. In particular colloidal carbon and two dispersing agents, known as R.D.A. and T.D.A. have proved effective. According to Kennedy, R.D.A., which is arylalkyl- sulphonic acid, and T.D.A., which is a mixture of tri-ethanol-amino salts and highly purified calcium salts of lignin sulphonic acid, were introduced as dispersing agents and catalysts to improve the properties of cement. These additives were introduced in the form of a solution in water, to the raw clinker feed of the tube mill in the proportion of about 0.06% by weight of the charge. Apart from the improvement in the setting properties of the cement, the output of the mill, for the same specific surface, was reported to be increased by 30-40%. The researches of Schweitzer and Craig into the grinding of cement clinker, in a laboratory ball mill, with carbon black as a grinding aid show a continuous increase in the rate of grind, or fineness attained in a given time, for additions up to about 1 % by weight of the feed. The rate of grinding thereafter fell with increasing amounts of the additive.
These results, which are summarized in the curve of fig. 9.1, show that the time required to grind to 85% through 325 mesh is reduced by 35% when compared with that required when no additive is used. The curve also shows that the optimum quantity of additive is fairly definite; small deviations from the optimum quantity having appreciable effect. This conclusion appears to be true for most additives.
As previously noted, quantities of additive as low as 0.08 % by weight of the solid present had a marked effect on the cleanliness of the mill surfaces, without producing noticeable colour deterioration of the product. The rate of
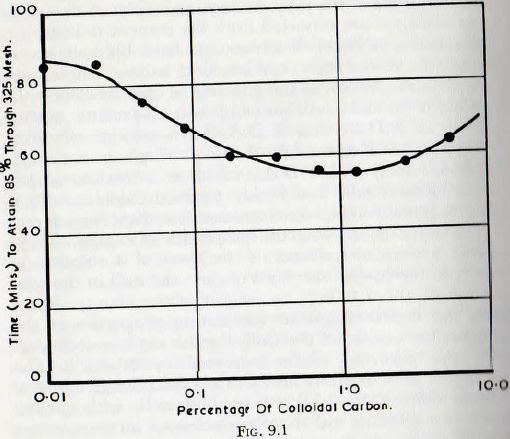
grinding is not, however, markedly improved by this small quantity of additive.
The work of Bond shows that the addition of a small amount of water, 0.88% by weight, brought about a reduction in the coating of the balls. A solution of T.D.A ., at about 0.13% by weight of the feed, however, brought about complete inhibition of ball coating. It was therefore concluded that the action of the additive in preventing coating of the ball charge was more important than effects arising from adsorption on the surface of the particles. This view is supported by the fact that the small amounts of additive which are sufficient to bring about a marked improvement in the rate of grinding, are sufficient to allow the formation of a monomolecular layer on less than 20% of the product surface. It is also supported by the fact that even large changes in the surface energy would produce but small proportional changes in the energy demanded by the whole grinding process (since the surface energy represents a very small fraction of the total energy demand of the process). It is also significant that the solid additives, mentioned earlier, all tend to inhibit ball coating; probably by imparting a greasy surface to the grinding media since they are all known to exhibit marked lubricating properties.
The effect of very small quantitites of water in preventing ball coating is known in other applications. For example, Manson states that it is well known in the paint industry that, in the grinding of dry-process enamels, the addition of as little as 0.0625 % of water can be effective in the prevention of ball and liner coating. Furthermore, the coating can also be removed from badly coated balls, under running conditions, by the addition of a few wet balls to the mill charge. Bond suggests that selective hydration of the particles at the ball surface might be the reason for the inhibition of coating by small quantities of water. Such a suggestion is, at the most, tentative.
The reason for such preferential hydration at the surface of the balls is not clear, but it must be remembered that, at the moment of impact, there is a considerable amount of energy dissipated and, doubtless, some of this could be used to bring about various chemical, or physico-chemical, reactions which demand an input of energy. This possibility is supported by an observation by Pryor, who found that copper ore was changed to a compound of a higher energy level at points where the balls of the mill rubbed the ore without breaking it.
There are other examples of the increase in the rate of grinding brought about by the use of additives in dry milling. For example, Waeser states that both R.D.A and ammonium salts are effective in the grinding of graphite and that oleic acid is an effective additive for the grinding of zinc blende. Gotte and Ziegler quote interesting results as to the milling of cement clinker, marble and glass in atmospheres of various polar and non polar substances. The tests were carried out in a vibrating mill, the additive being introduced in vapour form into the previously evacuated mill body containing the feed. The polar additives investigated were acetone, nitro-methane and water; the non-polar additives being carbon tetrachloride, benzol, hexane and petroleum ether.
These results can be summarized as follows:
- In the milling of clinker, the improvement brought about by the use of non-polar additives in place of an atmosphere of dry air, decreases with increasing fineness and increasing time of grind; the average increase in relative fineness being 10-20%. The polar additives show an increasing effectiveness with time of grind and fineness of powder; the most effective being acetone which led to improvements of 10-50% in the rate of grinding, relative to that carried out in dry air.
- Marble showed the same general characteristics but, in this case, water vapour, which led to a 60% increase in speed of grinding compared with a dry atmosphere was the most effective additive.
- For the grinding of quartz, polar and non-polar atmospheres led to about the same increase in the rate of grind.
- Glass proved anomalous in that, whilst non-polar additives effected some small increase in the rate of grind, polar additives produced a very definite decrease in the grinding rate. No definite explanation of this anomaly is forthcoming but it is possible that it might be connected with the strong adsorptive properties of finely divided glass powder.
Whilst it is realized that the tests of Gotte and Ziegler are not strictly relevant, in so far that they were carried out in a vibration mill, it is considered that they are of interest in that they are, as far as is known to the authors, the only tests carried out with a controlled atmosphere consisting of a surface active agent in vapour form. The method may well have applications for certain specialized batch dry-milling operations.
Turn now to the use of additives in wet milling. According to Berry, silicates, phosphates and arylalkyl- sulphonic acid are often used as additives in wet milling; the paint and colour industry, especially, offering a very wide field for the use of such agents (Anon).
Probably the most thorough investigation into the effects of additives in wet milling is that reported by von Szantho. In this work, the effect of varying percentages of flotation additives and of oleonic acid on the wet milling of quartz and limestone, in a laboratory batch ball mill, was investigated. In each case the characteristic product particle size, d, resulting from grinding for 30 minutes was estimated from the size frequency curves. The results, showing this characteristic particle size plotted against the percentage concentration by weight of the additive, are shown in Fig. 9.2. It is seen that, in each case, there is an optimum percentage beyond which the grinding rate decreases with any increase in the quantity of additive.
Von Szantho considers that the effect of the additive is primarily due to the lowering of the surface energy of the particles, arising from the adsorption of the additive, with consequent widening of the elementary cracks and fissures on the surface of the particles. As discussed previously, it is implicit in this conception of the operation of additives that their effect should become progressively greater with increasing fineness of grind. The undersize curves shown in Fig. 9.3 do in fact show that the effect of the additive is
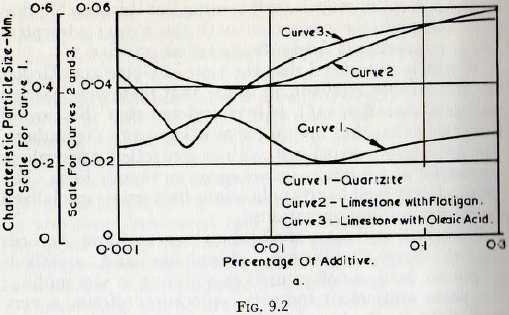
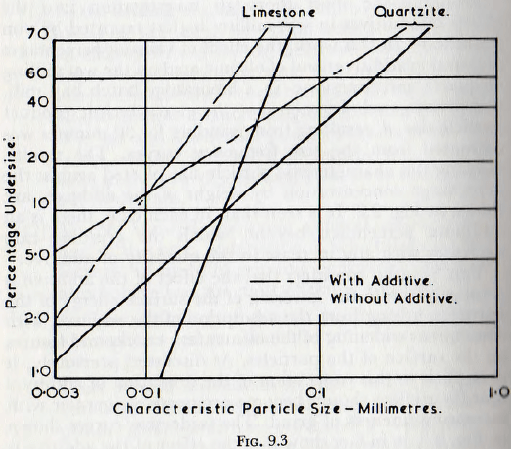
greater at the smaller particle sizes (for particles below about 60µ).
 |
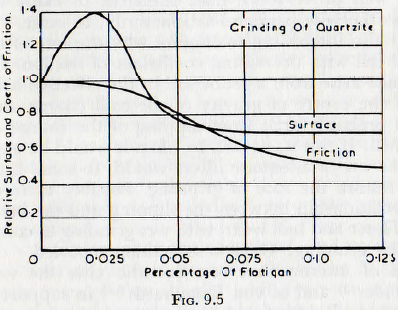 |
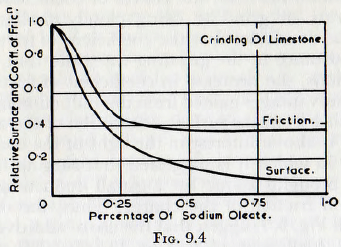
The decrease in the rate of grinding observed when the concentration of additive exceeds a certain value is tentatively explained on the basis of the lubricating effect of these substances at high concentrations. This explanation is supported by the data of Fig. 9.4 and Fig. 9.5, in which curves of surface produced and coefficient of friction are plotted against concentration of additive. These curves show that for concentrations of additive for which the coefficient of friction is high the rate of grinding is high, but when the coefficient of friction decreases the rate of grinding diminishes also; the curves of coefficient of friction and rate of grinding being closely similar. Thus, von Szantho concludes that the coefficient of friction is of major importance in the grinding rate, and that, at high concentrations, the decrease in coefficient of friction out weighs the advantages gained from the reduction in surface energy arising from the surface adsorption of the additive. This result is also of interest in the light of the contents of Chapter 7, in which it is suggested that surging in a mill might be brought about by a small reduction in the coefficient of friction of the charge. Thus, the curves of Fig. 9.4 and Fig. 9.5 suggest that the use of additives might reduce the coefficient of friction by 40-50% and this could well prove more than sufficient to cause a mill, which otherwise performs satisfactorily, to surge.
It is also interesting to enquire why the rate of milling should fall with decreasing coefficient of friction. Clearly this must arise from a reduction in the effective height of fall of the centre of gravity of the mill charge and this would probably arise from slipping of the charge on the mill shell. If this is, in fact, so, then it would be expected that the use of adequate lifters would, to some extent at least, restore the rate of grinding. Possibly there is also some relationship between the slipping and the increased rate of liner and ball wear, with wet grinding as compared with dry grinding, which is sometimes reported.
It is of interest that von Szantho cites the work of Rehbinder and of von Engelhardt in support of his conclusions. Rehbinder showed a correlation between the surface hardness of materials and their resistance to scratching with a fine point. It was also shown that there is a progressive decrease in the resistance to scratching with increasing quantities of additive adsorbed on the surface of the body, there being a close correlation between the resistance to scratching and the adsorption isotherm, with a minimum of resistance to abrasion being reached under conditions of complete adsorption. As much as 70% decrease in surface hardness was measured in this way, the maximum decrease corresponding to the greatest difference in polarity between the medium and the surface. It is suggested by Rehbinder that non-polar additives would be the most effective for hydrophilic materials, and polar additives should be used for hydrophobic crystals.
Engelhardt reports the work of Obreimoff, who measured the elastic work done in separating the lamina of mica and found that this was numerically equal to the theoretical surface energy of the new surface formed. When splitting of the lamina was carried out in the presence of surface active media, however, the surface exposed, for a given amount of strain energy, increased in proportion to the decrease in the surface energy caused by the adsorption. Engelhardt further conducted tests on the abrasive grinding of quartz with a silicon carbide disc, in the presence of various additives. It was found that polar and dipolar additives were very effective in reducing the resistance to abrasion. In particular small quantities of octanol or butyric acid in benzol or sodium silicate in water were effective in reducing wear resistance.
Skaupy has recently reported some outstanding results obtained from the milling of iron powder in various fluid media. Although the tests were carried out in a vibrating mill, they are included here on the grounds of their importance. It was found that the rate of grinding of iron was five times as great in glycol, and no less than twenty times as great in iso-amyl alcohol as in dry air. The tests revealed that dipolar additives were especially effective as grinding aids, as would be expected from surface energy considerations alone.
Although, in view of the more marked effect, the evidence points to a lowering of the surface energy as being an important factor in the operation of additives in wet milling, the evidence in the case of dry milling is more confused. The effectiveness of various materials with pro-show that for concentrations of additive for which the coefficient of friction is high the rate of grinding is high, but when the coefficient of friction decreases the rate of grinding diminishes also; the curves of coefficient of friction and rate of grinding being closely similar. Thus, von Szantho concludes that the coefficient of friction is of major importance in the grinding rate, and that, at high concentrations, the decrease in coefficient of friction outweighs the advantages gained from the reduction in surface energy arising from the surface adsorption of the additive. This result is also of interest in the light of the contents of Chapter 7, in which it is suggested that surging in a mill might be brought about by a small reduction in the coefficient of friction of the charge. Thus, the curves of Fig. 9.4 and Fig. 9.5 suggest that the use of additives might reduce the coefficient of friction by 40-50% and this could well prove more than sufficient to cause a mill, which otherwise performs satisfactorily, to surge.
It is also interesting to enquire why the rate of milling should fall with decreasing coefficient of friction. Clearly this must arise from a reduction in the effective height of fall of the centre of gravity of the mill charge and this would probably arise from slipping of the charge on the mill shell. If this is, in fact, so, then it would be expected that the use of adequate lifters would, to some extent at least, restore the rate of grinding. Possibly there is also some relationship between the slipping and the increased rate of liner and ball wear, with wet grinding as compared with dry grinding, which is sometimes reported.
It is of interest that von Szantho cites the work of Rehbinder and of von Engelhardt in support of his conclusions. Rehbinder showed a correlation between the surface hardness of materials and their resistance to scratching with a fine point. It was also shown that there is a progressive decrease in the resistance to scratching with increasing quantities of additive adsorbed on the surface of the body, there being a close correlation between the resistance to scratching and the adsorption isotherm, with a minimum of resistance to abrasion being reached under conditions of complete adsorption. As much as 70% decrease in surface hardness was measured in this way, the maximum decrease corresponding to the greatest difference in polarity between the medium and the surface. It is suggested by Rehbinder that non-polar additives would be the most effective for hydrophilic materials, and polar additives should be used for hydrophobic crystals.
Engelhardt reports the work of Obreimoff, who measured the elastic work done in separating the lamina of mica and found that this was numerically equal to the theoretical surface energy of the new surface formed. When splitting of the lamina was carried out in the presence of surface active media, however, the surface exposed, for a given amount of strain energy, increased in proportion to the decrease in the surface energy caused by the adsorption. Engelhardt further conducted tests on the abrasive grinding of quartz with a silicon carbide disc, in the presence of various additives. It was found that polar and dipolar additives were very effective in reducing the resistance to abrasion. In particular small quantities of octanol or butyric acid in benzol or sodium silicate in water were effective in reducing wear resistance.
Skaupy has recently reported some outstanding results obtained from the milling of iron powder in various fluid media. Although the tests were carried out in a vibrating mill, they are included here on the grounds of their importance. It was found that the rate of grinding of iron was five times as great in glycol, and no less than twenty times as great in iso-amyl alcohol as in dry air. The tests revealed that dipolar additives were especially effective as grinding aids, as would be expected from surface energy considerations alone.
Although, in view of the more marked effect, the evidence points to a lowering of the surface energy as being an important factor in the operation of additives in wet milling, the evidence in the case of dry milling is more confused. The effectiveness of various materials with pronounced lubricating properties such as carbon black, as an aid in the grinding of cement clinker, is much easier to explain on the basis of the inhibition of ball coating by means of the deposition of a layer of carbon on the balls. Furthermore, the small quantities of water which have been observed to remove the coating from a badly coated mill lends support for the idea put forward by Bond that, in this case, the particles are removed from the surface by a process of selective hydration, a surface phenomenon. However, the small quantities of water which are effective suggests that the adsorption occurs at the ball surfaces only. It thus appears that the surface phenomena involved are complex and that present knowledge is insufficient to give more than a very imperfect picture of the mechanism which is operative.
In conclusion, it is fair to say that any process of crushing must be influenced to a large extent by the nature of the atmosphere in which the operation is carried out. There is ample experimental evidence that in many cases, the energy or time of grind to a given fineness, and also the ultimate fineness of grind attainable, can be favourably modified by the introduction of a suitable additive into the mill. In view of the very large energy requirements for grinding, and in particular fine grinding, and also the low theoretical efficiency of the grinding operation as at present carried out, it would appear that the use of milling additives could effect substantial economies. It is also clear that the subject has not yet received the attention which its importance warrants, and it appears to be a field in which much interesting research remains to be done.
