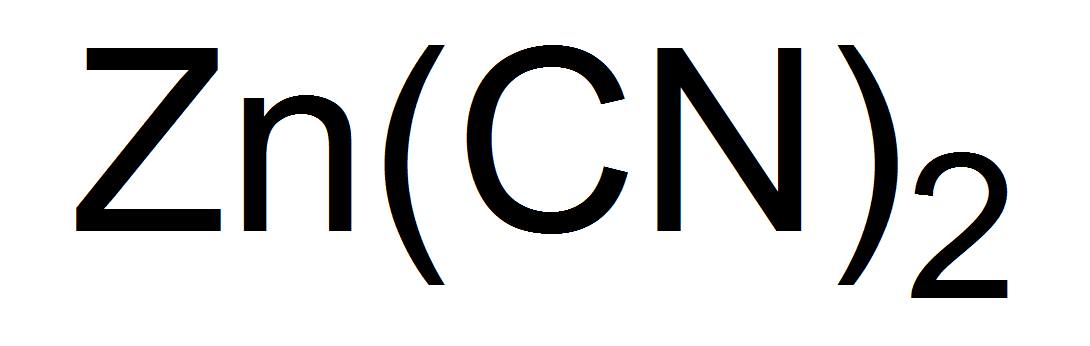 Dissolution of Precious Metals in Sodium Zinc Cyanide: The dissolving effect of sodium zinc cyanide on precious metals has been the subject of much discussion. According to one researcher, pure K2Zn(CN)4 in the presence of oxygen dissolves gold with the formation of gold potassium cyanide and zinc oxide. Another researcher compared the dissolving effects of KCN and K2Zn(CN)4 on gold. He found that a solution KCN when treated with excess zinc oxide had almost the same dissolving effect as the untreated KCN solution. When a large excess of alkali was added to both solutions the dissolving effect of the solutions increased by 25 to 30 %. This researcher concludes that the presence of zinc in cyanide solutions is not always detrimental.
Dissolution of Precious Metals in Sodium Zinc Cyanide: The dissolving effect of sodium zinc cyanide on precious metals has been the subject of much discussion. According to one researcher, pure K2Zn(CN)4 in the presence of oxygen dissolves gold with the formation of gold potassium cyanide and zinc oxide. Another researcher compared the dissolving effects of KCN and K2Zn(CN)4 on gold. He found that a solution KCN when treated with excess zinc oxide had almost the same dissolving effect as the untreated KCN solution. When a large excess of alkali was added to both solutions the dissolving effect of the solutions increased by 25 to 30 %. This researcher concludes that the presence of zinc in cyanide solutions is not always detrimental.
According to Rose and Newman, gold is dissolved by solutions of Na2Zn(CN)4 although the double cyanide remains unaltered as long as any simple cyanide is present. A solution of Na2Zn(CN)4 containing lime and with free access of oxygen is almost equivalent in dissolving power to one of equal NaCN or KCN strength. The same authors state that when zinc dust is used to precipitate copper from cyanide solutions a rapid and deleterious accumulation of zinc in solution soon occurs. The sodium zinc cyanide formed is only a weak solvent for gold, but the addition of lime* or soda liberates free cyanide and thereby raises the efficiency of the solution.
White investigated the effect of Na2Zn (CN) 4 on the dissolution of plates consisting of gold 87%, silver 11.3% and base metal 1.7%. The rate of dissolution increased up to a strength of 0.027 % KCN equivalent.
Hamilton tested the dissolving effect of K2Zn(CN)4 solution equivalent to 0.3% KCN on pure, precipitated, dried and pulverized silver sulphide. 24 hours agitation in an open bottle with this solution had almost no dissolving effect on the silver. When various amounts of free cyanide were added to solutions of the double cyanide, the dissolution of silver was in every case closely proportional to the quantity of free cyanide present, and corresponded with the weights of silver dissolved by solutions containing similar strengths of free cyanide but with the double cyanide absent.
Leaver and Woolf investigated the effect of zinc minerals in ores on gold and silver extraction. They summarize their findings as follows: .
“The various zinc cyanide compounds formed in the mill solutions are only weak solvents for the precious metals, even if the usual titration with silver nitrate shows excess free cyanide; that is, if considerable zinc is present in the solution, the titration for free cyanide is misleading as to the efficacy of the solution as a solvent for silver and gold.
“The addition of excess lime or caustic soda improves the extraction with solutions containing zinc by liberating free cyanide from the double salt.”
“If most of the cyanide present in a solution is combined with zinc, more free cyanide must be added to this solution than to a fresh solution for the two solutions to have equal activity as solvents.”
“The deleterious effect of zinc in a solution may be entirely overcome by using solutions excessively strong in free cyanide. The continued addition of the required amount of free cyanide would soon result in an excessive amount of cyanide being tied up in the mill solution. Furthermore, strong cyanide solutions consume more zinc during the precipitation of the precious metals, thereby, increasing zinc consumption and causing additional fouling of the solution. It is, therefore, advisable when the mill solution becomes foul with zinc either to remove the zinc and regenerate the cyanide or to discard the solution.”
According to Anderson a zincate cannot exist in the presence of free potassium cyanide because the following reaction takes place:’
K2ZnO2 + 4 KCN + 2 H20 = Zn(CN)2*2 KCN + 4KH0
Some state that the addition of free cyanide to a solution containing zincate must first convert the zincate of alkali into the double salt before the solution can contain any free cyanide at all.
Thus, a certain amount of contradiction is evident in the conclusions arrived at by various investigators regarding the solvent effect of double zinc cyanides on precious metals. Probably some of these differences of opinion arise from the method used for assessing the true free cyanide content of solutions containing double zinc cyanides. In some cases it has been assumed that the silver nitrate method for the determination of free cyanide is equally valid when applied to solutions containing sodium zinc cyanide. This, however, as many authorities have stated, is open to question. As pointed out earlier, when titrating a solution containing sodium zinc cyanide for free cyanide with silver nitrate, the value obtained for free cyanide varies according to the alkalinity of the solution. With a high lime solution and using potassium iodide as indicator, practically all of the cyanide in the zinc complex reported as free cyanide.
Other differences of opinion in connection with the solubility of precious metals in sodium zinc cyanide have probably arisen from the variety of systems employed to investigate this phase of cyanidation and also from the failure of many investigators to define clearly those systems. For example, in many cases the ratio of total cyanide to zinc in the sodium zinc cyanide solutions tested was not given; in this connection, when sodium cyanide reacts with oxide zinc minerals, sodium hydroxide is formed in addition to sodium zinc cyanide and this sodium hydroxide dissolves oxide zinc minerals to form soluble zincates. The result is to lower the ratio of total cyanide to zinc in such solutions. In other cases no clear differentiation was made between dissolution and rate of dissolution of the precious metals. Another factor that has not been given due consideration is the different mechanisms of the reactions for the dissolution of gold as compared with those for silver minerals. With gold, the rate of dissolution is governed by the maximum solubility of oxygen in the cyanide solution and, under ideal conditions, there is no advantage as regards rate of dissolution in having cyanide solutions stronger than 0.0098 % NaCN, whereas, with silver sulphide minerals, at least two factors affect the rate of dissolution. One is strength of solution; the stronger the solution, the greater will be the tendency for the reaction Ag2S + 4 NaCN <–> 2 NaAg(CN)2 + Na2S to go to the right. The other is the rate of oxidation of sodium sulphide and its decomposition products; the more rapidly these are oxidized, the more rapidly will the silver dissolve. Again, neither oxygen nor strength of solution may enter into the rate of dissolution of non-sulphide silver minerals such as Ag20 and AgCl; these minerals dissolve very rapidly in sodium cyanide solutions without using oxygen and it is conceivable that they would dissolve equally as rapidly in sodium zinc cyanide solutions.
The rate of dissolution of precious metals in solutions of sodium zinc cyanide is probably related closely to the dissociation of this complex, and the extent to which this dissociation proceeds under various conditions. It is generally accepted that sodium zinc cyanide dissociates as follows:
Na2Zn(CN)4 <–> 2 Na+ + Zn(CN)4= <—>
2Na+ + 2CN- + Zn(CN)2
When a solution of sodium zinc cyanide is prepared by treating an excess of zinc cyanide, Zn(CN)2, with sodium cyanide and filtering off the excess zinc cyanide, the clear solution often shows a white opalescence of zinc cyanide after standing a while. Apparently after some of the CN ions have been lost, by oxidation or hydrolysis for example, a corresponding amount of zinc cyanide is thrown out of solution; thus equilibrium is restored.
In the presence of sodium hydroxide the dissociation proceeds thus:
Na2Zn(CN)4 + 4NaOH <–>
6 Na+ + 4 CN- + ZnO2= + 2 H+ + 2 OH
If sufficient sodium hydroxide is present sodium, cyanide, and zincate ions would be the significant dissociation products; no zinc cyanide would be precipitated as this compound is soluble in sodium hydroxide forming sodium zinc cyanide and sodium zincate. Whether dissociation in this case proceeds to a greater degree than when no sodium hydroxide is present, is a debatable question. Another moot point is whether lime acts in the same manner as sodium hydroxide. If lime forms calcium zincate under these conditions, it is probable that it would be much less soluble than sodium zincate and that the dissociation of sodium zinc cyanide in the presence of calcium zincate would be affected accordingly.
Another factor which would undoubtedly affect the dissociation of sodium zinc cyanide would be the presence of zincate ions resulting from a different reaction as, for example, the reaction between oxide zinc minerals and alkali. These zincate ions would repress the dissociation of sodium zinc cyanide in accordance with the principles of the common ion effect.
In summation, it may be stated generally that all mill solutions contain sodium or calcium zinc cyanide. The amount will vary according to the amount of zinc used for the precipitation of the precious metals, and according to the amount and the types of zinc minerals which might occur in the ore. Under ordinary conditions of alkalinity in the mill solutions, it is probable that the greater part of the cyanide thus combined with zinc will report as free cyanide by the silver nitrate titration. In cases where a drop in the efficiency of a cyanide solution is suspected even though the titration indicates adequate free cyanide, it is advisable to determine the zinc content of the solution, and to determine the total cyanide content, preferably by distillation. If there are close to 3 parts by weight of NaCN to 1 of zinc (corresponding to the complex Na2Zn(CN)4), then the only free CN ions present are those resulting from the dissociation of this complex. When zincate is present in the solution in addition to Na2Zn(CN)4, the ratio of NaCN to zinc will drop below 3 to. 1 depending upon the amount of zincate. The dissociation of CN ions will decrease accordingly and so will the dissolving efficiency of the solution. In some cases, as for example in the cyanidation of an ore containing fine free gold, the complex Na2Zn(CN)4 might be adequate for maximum gold extraction provided that too much zincate is not present. In other cases, notably in the treatment of ores containing silver sulphide minerals, where solution strengths in excess of 0.1 % free NaCN are required, the complex Na2Zn(CN)4 might be totally inadequate, and cyanide in excess of 3 parts of NaCN to 1 of zinc would have to be maintained in the solution to insure maximum extraction.
