Table of Contents
- Acknowledgments
- Mineralogy
- Leaching Technology
- Heap Leaching Ore Preparation
- Dump Leaching Ore Preparation
- In Situ Leaching Ore Preparation
- Leaching Process
- Recovery
- Leaching Operations
- Arizona
- California
- Colorado
- Idaho
- Montana
- Nevada
- New Mexico
- South Dakota
- Permitting Regulations
- Federal Regulations
- State Regulations
- Leaching Problems and Research
- Percolation
- Temperature
- Calcium Salt Scale
- Research On Novel Solution Mining Methods
- Summary
Climbing gold and silver prices during the 1970’s awakened dormant interest in mining these metals in many districts. Since gold and silver deposits frequently can be profitably mined by small operators, a mix of mining activity has been created, comprised of large and small companies, both experienced and unexperienced. A key factor in the profitability of small mining operations is the advent of an alternative to conventional mining and milling operations—solution mining (leaching). Basically gold and silver leaching involves spraying a cyanide solution on the ore to dissolve the metal values, collecting the solution containing the dissolved metals, and recovering the metal from the solution. By eliminating milling, leaching reduces capital cost and startup time for new operations. Operating costs are likewise significantly lower. The disadvantages of leaching are lower recovery and greater difficulty in controlling the cyanidation process.
There are three types of leaching systems—heap, dump, and in situ; “vat” leaching accompanying conventional milling operations is not considered in this publication. If ore is mined or if it is gathered from old mine waste-rock piles and hauled to specially prepared pads lined with clay, tar, or Hypalon for leaching, the method is “heap” leaching (figs. 1-2). The rock is frequently crushed before being placed on the pad.
If mine waste-rock piles or dumps are judged to contain sufficient mineral value to justify leaching and the solutions can be controlled without
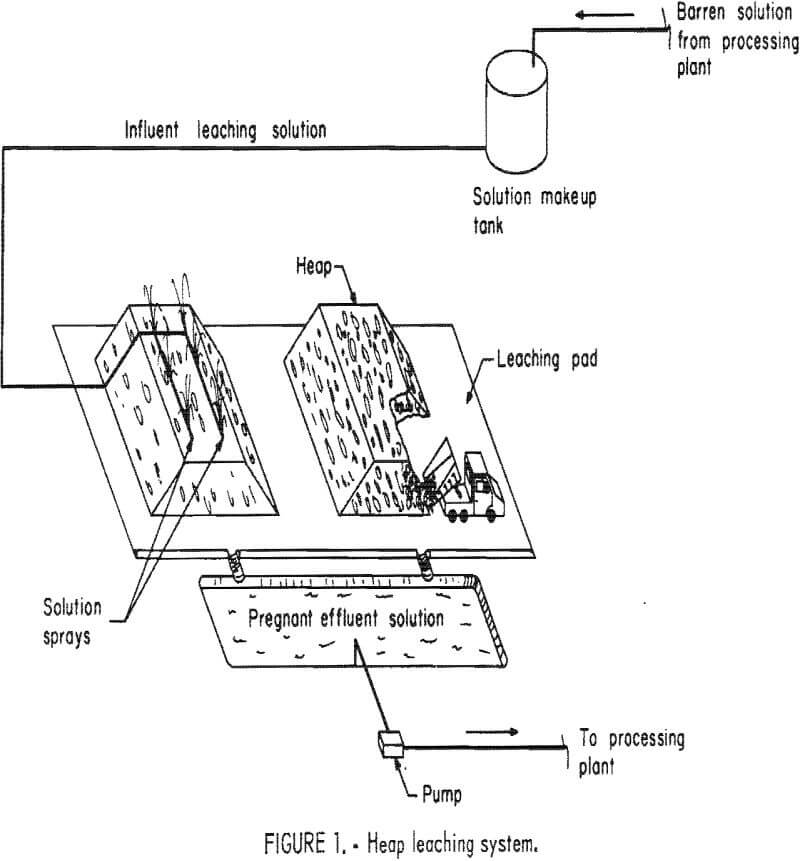

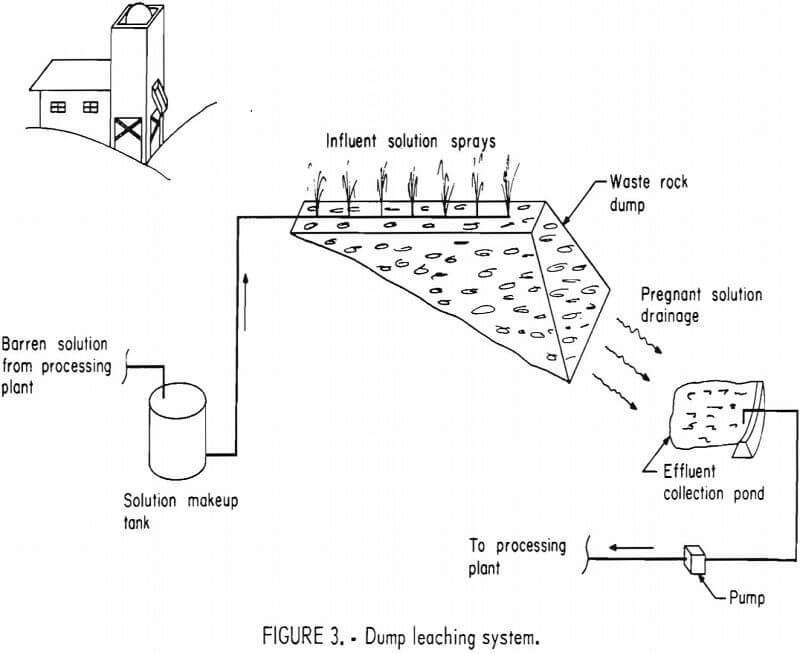
appreciable losses, the pile is “dump” leached without any preparation (fig, 3), Although this technique is rarely used for leaching gold and silver, it is common in the copper industry.
Finally if the ore is broken and left in place or if it conducts fluids flow without blasting, it can be leached “in situ” or in-place (fig- 4). An exposed ore body can be leached in situ by spraying solution on the surface and collecting it in recovery wells after it has percolated down through the ore. For buried ore bodies, the solution must be injected into the formation through injection wells and recovered from adjacent recovery wells. Although copper and uranium have been leached in situ, there have been only sporadic attempts to develop such operations for leaching gold and silver. One attempt to in situ leach gold at the Ajax Mine near Victor, CO, is described later.
Since many operators are considering leaching gold and silver for the first time or are experiencing problems in establishing leaching operations, this report summarizes leaching principles and practices, discusses problems that may be encountered, and lists sources of additional information in a bibliographic appendix.

Acknowledgments
The authors wish to express appreciation to the following mining companies that provided engineering data on their leaching experiments or operations:


Mineralogy
Since many good references are available on the geology of gold deposits, such information is not provided herein. Of particular importance however, in evaluating the leachability of gold-silver deposits is their mineralogy.
Gold is usually deposited as native or free gold associated with pyrite. Occasionally, as at Cripple Creek, CO, the gold is deposited as a telluride. Various heavy metal compounds are frequently associated with the gold.
Silver is usually deposited in its compound form. Besides native silver (Ag), those minerals containing leachable silver are argentite (silver sulfide, Ag2S), cerargyrite (silver chloride, AgCl), embolite (AgCl, AgBr), and bromyrite (silver bromide, AgBr). Other silver minerals are not readily leachable.
Ore can be economically leached at grades about an order of magnitude lower than they are commonly milled. Current leaching operations are producing gold from ores containing as little as 0.03 oz/ton with cutoff grades down to 0.01 oz/ton. Most silver leaching operations produce from ores grading 1 to 4 oz/ton. The easiest ores to leach are those that have been weathered or oxidized, liberating the gold or silver from pyrite or other encapsulating minerals.
A variety of mineralogical conditions can hamper or prevent leaching of an ore. For example, deposits that contain organic carbon are not suitable because the carbon prevents much of the gold from dissolving and adsorbs any dissolved metal before the leach solutions are recovered. Other refractory ores are those in which the gold or silver is totally encased by an impervious matrix material such as quartz so that the leaching solutions cannot contact the metal. Copper, cobalt, and zinc in the ore may preferentially take the place of gold and silver in the leaching reaction and greatly reduce the reaction with the desired metal.
Some ores, such as tellurides or those containing arsenopyrite or antimony, must be roasted before they can be leached with cyanide and so are not amenable to heap leaching. Although other leaching solutions have been investigated for use with telluride deposits, no commercial heap leaching operations have resulted.
Pyrrhotite is another mineral that complicates leaching. Decomposition of pyrrhotite in cyanide produces ferrocyanide, which removes free cyanide from solution and prevents its reaction with the gold or silver. This decomposition also removes oxygen from the solution, which further decreases the reaction with gold and silver.
If manganese occurs with silver ores, its higher order oxidation products can form refractory compounds of silver and manganese. Many silver deposits were left unmined throughout the Western United States because of this particular problem. Some of these deposits may be amenable to dual leaching, first with aqueous SO2 to recover manganese, followed by neutralization and leaching with cyanide to recover silver.
Clay minerals in the ore pose a major problem in leaching operations. The clay particles block the leaching solution’s flow through the ore and isolate large portions of ore from the solution. Some heaps contain so much clay that the solution perches on top with negligible downward percolation.
Leaching Technology
Heap Leaching Ore Preparation
Ore preparation for heap leaching consists of
- preparing an impervious pad,
- mining or gathering ore from dumps,
- crushing the ore (optional), and
- placing the ore on the pads.
Pads are usually situated in flat terrain that is graded to provide gently sloping surface for drainage (2 to 5 pct). Next the pad site must be lined to prevent solution seepage losses. If plastic liners are used, the ground is covered with a layer of sand or tailing that is rolled to provide a cushion. The liner sections are laid out and cemented together, then covered with sand to provide additional cushioning. Heaps that are subjected to much vehicular traffic are often lined with asphalt. Some pads are merely lined with a thick clay or tailing layer that becomes essentially impervious when wet. A dam or berm with a drain is constructed on the downstream end of the pad to direct the solution into a holding pond.
Size distribution of ore placed on heaps ranges from run-of-mine to minus ¼ in (6 mm). Many miners crush the ore to significantly improve total recovery and recovery rates. Operating costs are, of course, higher when ore is crushed. Before an operator selects a particular particle size, the ore should be tested to determine the trade-off between mineral recovery and crushing costs at several sizes.
Ore is spread on the prepared pads by scrapers, front-end loaders, trucks, and bulldozers; conveyors and stackers can also be used. A unique gantry system has been employed at one operation in New Mexico. Since solution percolation into a heap is severely affected by any packing from driving on the surface of the heap, the specific technique by which the ore is placed in heaps profoundly influences precious metal recovery. Techniques that eliminate traffic on the implaced ore are strongly urged.
Each layer, called a lift, is usually placed 5 to 10 ft (2 to 3 m) deep; the range encountered at current operations was 2 ft (1 m) to 20 ft (7m). Although it has long been felt that after solutions percolate through a heap greater than about 10 ft (3 m) deep, they may become oxygen deficient, the rate of oxygen depletion has not been accurately measured. Recent experiments with hydrogen peroxide additives have shown no increased metal recovery, which would indicate that the critical oxygen content is not as high as originally envisioned.
After the first lift has been leached, either it is removed from the pad, or the next lift is placed on top of it so that subsequent applications of leaching solutions will percolate through both. A third and fourth lift can be added later. Operations using fine ore size generally leach with only one lift because at the end of the leaching cycle the ore is virtually depleted and may as well be removed. Operations with larger sized ore particles that take longer to leach generally use multiple lifts. The solution can then pick up extra mineral values from the lower lifts until they are depleted without wasting valuable pad space.
Placing ore on heaps so as to prevent surface packing is a problem. If vehicular traffic packs the surface into a hard pan, the leaching solution will not percolate uniformly downward; in fact, stagnant paddles or ponding on the surface may occur. Although packed material can be loosened with a ripper, several companies are considering conveyor systems to eliminate vehicular traffic on heaps. Pushing the material into place with a front end loader after it is dumped on the pad also minimizes surface packing.
Where clay in the ore causes percolation problems, a relatively new technique of agglomerating the fines can be used to prepare the ore. Devised by the Bureau of Mines’ Reno Research Center, the technique dramatically improved percolation rates, prevented channeling, and improved leaching rates in pilot tests. Several companies have used the technique in full-scale operations since 1980. At one of these sites, recovery improved from 37 to 90 pct as a result of agglomeration. The technique involves mixing the dry crushed ore with 5 to 15 lb/ton (2.5 to 7.5 g/kg) portland cement, wetting with 8 to 16 wt pct water, mechanically tumbling the wetted feed to effect agglomeration, and curing the agglomerated material for 72 h prior to leaching. The lime in the portland cement reduces the amount of lime that must be added to the leaching solution to maintain the proper pH; see the following section on leaching solutions. Even greater benefits accrue if, instead of using water, the cement-ore mixture is wetted with relatively strong cyanide solution during agglomeration. This cyanide solution can then begin precious metal dissolution while the pellets are curing so that the heap can be leached with water that can be recirculated throughout the leaching cycle. Preliminary tests resulted in reduced cyanide consumptions and reduced leaching time.
Dump Leaching Ore Preparation
Although waste rock from either underground or open pit mines is of too low a grade to warrant conventional milling, some gold or silver may be recovered by leaching it. If dumps are leached without additional ore preparation or transporting the rock to prepared pads, the operation is termed a dump leach. Caution must be exercised in selecting dumps to insure that no leach solutions can escape into ground water or surface water drainage. A heavy clay soil underlying the dump is necessary to prevent solutions from percolating to the ground water. Dams are generally constructed to trap and hold the leach solutions.
In Situ Leaching Ore Preparation
There are no commercial-scale in situ gold or silver leaching operations In the United States. If such leaching is undertaken, ore will probably be prepared by blasting the formation to rubblize it. For shallow deposits [less than 300 ft (100 m)] the deposits would be rubblized with explosives placed in vertical holes patterned after techniques used for in-place copper leaching. Deep deposits would require blasting methods similar to those employed at conventional underground mines. For instance, if a deposit would be developed by a certain stoping configuration for conventional mining, the same configuration would likely be the most efficient for preparing the ore for in situ leaching. The only difference would be that in conventional operations all of the rock from development openings and ore from the stopes is hauled to the surface, whereas in leaching operations only the 20 to 25 pct of this material would be hauled to the surface to provide “swell” space for the rubblization blast.
Certain shallow underground mines in well-fractured formations may be amenable to in situ leaching without any ore preparation. Access to the deposit would be gained via the mine development openings; solution injection and recovery wells could be drilled from any of these openings at possible cost savings (by eliminating drilling through barren overburden). Placer gold deposits would probably be permeable enough to permit leaching from vertical wells without blasting.
Leaching Process
Leach Solutions
Although it is possible to leach gold and silver with several types of solutions, all current operations use sodium cyanide (NaCN), mixed in water at strengths of about 1 lb/ton (0.5 g/kg) of solution, or 0.05 pct. Strengths encountered in current operations range from 0.3 to 5.0 lb/ton (0.15 to 2.5 g/kg). The higher strengths are generally used on ores with high silver content.
When the cyanide solution contacts free gold or silver, leaching occurs according to the following reactions:
2Au + 4NaCN + O2 + 2H2O → 2NaAu(CN)2 + H2O2 + 2NaOH
and 4Au + 8NaCN + O2 + 2H2O → 4NaAu(CN)2 + 4NaOH.
The reaction depends strongly on oxygen, which is added by bubbling air through the solution and/or by spraying the solution onto the heaps.
When other silver minerals are leached, the silver similarly combines with the cyanide ion except that silver chloride apparently will combine without oxygen; for instance,
AgCl + 2NaCN → NaAg(CN)2 + NaCl.
Leach solutions are effective at pH 9.5 to 11, although both lower and higher alkalinities have been successfully used. Lower pH may result in decomposition of the cyanide by hydrolysis or by reaction with carbon dioxide in the air. Low pH can also permit gasification (and hence loss) of the cyanide if the solution contacts natural ground acids. Conversely, excessively high alkalinity seems to retard the reaction. The desired pH is maintained by adding lime (CaO) or caustic soda [sodium hydroxide (NaOH)] at about 0.5 to 1.0 lb/ton (0.25 to 0.5 g/kg) of solution. Although caustic soda is more expensive, it reduces maintenance problems caused by lime scale. Some operators claim that both the cyanide concentration and pH should be higher for silver ores than for gold ores, but no trend could be discerned from the engineering data available.
Solution Distribution
Leach solutions are pumped from a mixing pond to the distribution site after the cyanide and lime (or caustic soda) have been added. Upon reaching the site, the solution travels through a grid of distribution lines (usually ¾- to 2-in- diam plastic tubing) deployed across the top of the heap or dump. Spray nozzles, sprinkler heads, or wriggler tubing connected at various intervals into the main distribution line apply the solution.
Fixed-spray systems are the cheapest and easiest to install; some operators merely punch holes in the distribution lines at fixed intervals to create a spray (fig. 5). Although these require little maintenance, channeling is a common problem. Other operators may attach short feeder lines connected to fixed lawn-type sprinklers, but these do not distribute the solution as uniformly as do other systems and frequently result in channeling.
The most common solution distribution system is the oscillating lawn sprinkler, usually referred to as the rainbird sprinkler (fig. 6). This sprinkler gives a more uniform coverage than fixed sprays. These sprinklers are, however, susceptible to calcium salt scale, which restricts the flow. When the scale severely restricts solution flow, the sprinklers must be removed and soaked in hydrochloric acid to dissolve the calcium salts. Some operators have experimented with modifying the sprinkler orifice in an attempt to reduce this problem and to distribute the solution without the ring of more concentrated sprinkling that seems common from off-the-shelf sprinklers. Sprinklers are generally operated


with 20 to 40 lb/in² (0.14 to 0.28 x 10 6 Pa) line pressure, which yields a radius of coverage averaging 35 to 50 ft (11 to 15 m).
The so-called Bagdad wiggler, named for its development at Cyprus Bagdad Mine in Arizona, has recently become popular. A wiggler is constructed by cutting a 9-in (23-cm) long segment of ¼-in (6-mm) thick-walled gum rubber tubing and forcing one end over a hose connection attached to the feeder lines (fig. 7). As the solution passes through the tubing, the free end wiggles and sprays the solution around in a circle. Wigglers are generally placed on 10-ft (3-m) centers. If the wiggler flops in a figure 8 instead of a circular path, the ends are sliced with three short spiral cuts. Proponents argue that wigglers have better distribution patterns than sprinklers. Maintenance is relatively easy; when calcium salt scale appears in the lines, the wiggler can be stretched and shaped to dislodge the buildup. Wigglers are operated at around 20- to 40-lb/in² (0.14 to 0.28 x 106 Pa) line pressure.
An even newer but similar solution distribution technique employs the use of a wobbler. Wobblers are made by attaching lengths of thin plastic (Tygon) tubing to the solution feeder lines. At normal operating pressures the wobblers will produce a 4-ft (1.2-m) high spray with a radius of 9 ft (3 m).
At least two operators are applying solutions by ponding (fig. 8). Leach solutions are directed over the top of the heaps, where berms and dams hold the solution in a pond. The pond is kept several inches deep as the solution percolates downward. A major disadvantage Is that appreciable clay-size particles in the heap or dump material will thwart percolation and will promote channeling drastically. Another problem with ponding is maintaining sufficient oxygen in the solution for efficient dissolution of gold and/or silver.
Regardless of the distribution method, after percolating through the ore the solution drains off the pad into a holding pond (fig. 9). The pond is lined with



plastic sheet or a clay layer to prevent seepage. The pond functions both as a surge pond and as a settling basin for particles contained in the pregnant solution. Settling rates may be increased by adding a flocculant.
Solution application rates vary among operators. Generally the rate should be kept below that which would cause the solution to pond on the surface and then channel through the ore heap or dump. Experiments at several sites have indicated optimum leaching rates of around 0.005 gal/min·ft-² (3 x 10 -4 mL/s-cm-²) of pad area; rates at commercial operations range from that figure up to 10 or 20 times higher. Recovery plants have been established to handle throughput rates of 50 to 3,000 gal/min (3 to 18 L/s).
Recovery
Comparison of Techniques
Gold and silver are recovered from the pregnant solutions by precipitation with zinc dust or by adsorption on activated carbon in the form of charcoal. Each method had advantages and disadvantages. Selection of a specific system will depend on the specific conditions at a leaching operation and the facilities already available.
Most of the conventional mill circuits were based on the Merrill-Crowe system for precipitation of gold and silver by zinc dust. Although the capital expenditure required to establish such a system has been high, there are now on the market, small packaged plants designed and priced for small leaching operations. Costs are reduced in these systems by eliminating the high-cost, countercurrent decantation circuit used in a conventional milling circuit.
Where an old mill circuit can be converted to handle a leaching system, zinc precipitation usually costs less than charcoal adsorption. The low cost of zinc also favors zinc precipitation systems. One hundred pounds of zinc dust costs about $35 and will precipitate 600 to 800 oz of gold, while the amount of activated carbon needed to adsorb about the same amount of gold (about 1 ton) costs $2,500. Although the zinc is expended, 70 cycles must be achieved with the charcoal to balance the material costs. Zinc precipitation also does not require the capital and operating costs of a charcoal-stripping plant and kiln for reactivating the charcoal.
If more silver than gold occurs in the leach solution, zinc precipitation offers a further advantage because with charcoal adsorption, a large volume of high-cost material is tied up adsorbing a lower value metal. The problem of recovering silver in leach solutions has been somewhat alleviated by recent Bureau research, which has demonstrated that silver can be effectively precipitated from the leaching circuit as Ag2S by adding sodium sulfide; the solution can then be passed through the carbon adsorption columns to recover the gold.
Carbon adsorption systems are, however, becoming increasingly popular, and they do have several advantages over zinc precipitation systems. The primary advantages of carbon adsorption are that the systems can adsorb metals from solutions that contain suspended solids and from solutions that contain low metal concentrations (less than 0.05 oz/ton of solution). Charcoal adsorption also does not require a filtration system nor a de-aeration tank and pump. Environmental damage from zinc salts is avoided.
Zinc Precipitation
A generalized flow diagram for precipitating gold and/or silver with the zinc system is shown in figure 10. First, pregnant solutions are pumped from the holding pond to the processing plant. Since suspended particles will coat the zinc and retard its reaction, the first step of the process is to filter the solution (usually with plate and frame filters). The filters are often coated

with diatomaceous earth to help remove the suspended particles and prolong filter life.
Gold and/or silver precipitation also depends on complete removal of dissolved oxygen. Although this is usually achieved by a Crowe-type vacuum tank and pump, air can also be removed by sand filters and ceramic baffles. Failure to remove the oxygen permits the precipitated gold and silver to be redissolved. Even more importantly, any oxygen present when zinc dust is added forms hydrated zinc oxide, which coats the zinc and prevents further reaction with the solution.
Gold and silver precipitation are improved by adding lead acetate or lead nitrate to the solution. The lead forms a bond with the zinc that has a greater galvanic activity than the zinc alone and precipitates the precious metals faster. The lead acetate or nitrate added is about 10 wt pct of the zinc dust. Care must be taken to prevent coating the zinc with too much lead, which retards the galvanic action. Silver in the solution can form the same galvanic couple as does lead with zinc so that adding lead acetate is often unnecessary for leaching solutions containing appreciable silver.
Zinc dust is fed into the solution by a screw-type feeder and cone arrangement a Precipitation proceeds according to the following reaction (12) for gold and silver:
NaAu(CN)2 + 2NaCN + Zn + H2O → Na2Zn(CN)4 + Au + H + NaOH.
Since the zinc should precipitate gold or silver 1 mol/mol, approximately, equivalent amounts of zinc should be added as there are precious metals in the solution. In actual practice, anywhere from 10 to 100 pct more zinc is added than theoretically should be needed. Enough free cyanide must be present to dissolve the zinc so that it can replace the gold in the alkaline compound and so that the resulting zinc alkaline compound remains in solution. The accompanying liberation of hydrogen is necessary to create the reducing conditions for precipitation.
After the zinc has been added to and mixed with the leach solution, the solution is forced through Merrill-type pressure filters. This leaves behind the gold and/or silver plus impurities. The precipitate is removed from the filter and dried for smelting.
For either all-gold or all-silver precipitates , a flux is added and the precipitate is smelted for pouring. If both gold and silver are present in significant amounts the precipitate can be fluxed and smelted for sale as a dore bullion. If separating the gold and silver is desired, silver can be dissolved from the filtered precipitate with nitric acid and electrowon from the solution. The remaining precipitate is smelted for obtaining its gold. As an alternative, gold can be separated from the precipitate by dissolving it in aqua regia and then recovered by precipitating it from the aqua regia with oxalic acid or by electrowinning it.
Charcoal Adsorption
Charcoal adsorption systems for recovering gold and silver (fig. 11) have been described in great detail. First, the pregnant leaching solution is pumped from the holding pond to the processing plant where it flows through three to five carbon columns. Each column contains granular activated carbon manufactured from coconut shells (minus 6 plus 16 mesh or minus 12 plus 30 mesh). The pregnant solution can be percolated down through a fixed bed of the charcoal in each column, or it can be directed upward through the charcoal at a rate that maintains the charcoal in a suspended state (fluidized). Although columns with fixed beds require less charcoal for the same amount of solution, they are more susceptible to plugging from particles carried in the solution than are those with fluidized beds. Most commercial operations, therefore, use fluidized beds. Flow rates required to maintain a

fluidized condition range from 15 to 25 gal/min·ft-² (1 to 1.7 L/s·cm-²), depending on the size of activated carbon used.
The columns of charcoal are arranged in countercurrent series so that the fresh solution first enters the column that contains the charcoal with the most adsorbed precious metals. As the solution flows through the charcoal, gold and silver are adsorbed onto its surface. The solution passes through columns containing charcoal with successively less adsorbed metals until it emerges as a barren solution from the last one. After emerging from the last column, the barren solution is pumped to the makeup pond.
When the front column of carbon (or a portion of it) reaches its desired loading capacity, the carbon is removed for stripping. An identical amount of carbon is then removed forward in each column, and a fresh charge is added to the last. Charcoal loading formerly ranged from 400 to 800 oz of metal per ton of charcoal (14 to 27 g/kg); a recent trend towards lower loading and more frequent stripping commonly results in loading levels of 150 to 250 oz/ton carbon (5 to 9 g/kg). Factors that affect charcoal loading are solution grade, flow rate, gold-to-silver ratio of solution pH, charcoal type, and impurity concentrations.
The loaded charcoal removed from the columns must be stripped of the precious metals. Stripping is accomplished with a hot caustic soda solution. The traditional Zadra process involves soaking the loaded charcoal at 93° C in a 1.0 pct NaOH-0.1 pct NaCN solution for 24 to 48 h. By adding 20 pct ethanol or methanol to the solution, the time can be reduced to 5 or 6 h. The stripping time and chemical consumption can be further reduced by pressure stripping with a 0.4-pct NaOH solution (without NaCN) at 150° to 200° C and 50 to 90 lb/ in (0.3 to 0.6 10 Pa).
Gold or silver are next removed from the stripping solution by electrowinning. Typical electrowinning cells contain a stainless steel anode plus a stainless steel wool cathode for plating the metal.
The steel wool cathode is usually packed to a density of 1 lb/ft (16 kg/m ) and provides a cathode surface area of 10 ft/lb (2 m /kg). Operating voltages run from 2.5 to 3.5 V. Significantly higher voltages can break down the solution and generate hydrogen or ammonia gas, which blocks the plating action. Current ranges from 20 to 30 A, which provides a current density of 3 to 3.5 A/ft at the cathode. The solution should be retained in the cell at least 15 min, preferably 30, to win the gold and silver.
The stripped carbon is regenerated by heating in a kiln or chamber at approximately 700° C (1,300° F) in a reducing atmosphere such as steam. Although carbon can be used two or three times without regeneration, it is simpler to regenerate it each time rather than trying to keep track of which batch needs regenerating. The life expectancy of carbon has not been well documented. Some operators have experienced a 25-pct reduction in adsorptive capacity after eight or nine cycles, while others have found only a 33-pct reduction after 8 or 9 yr of continuous use. A few operators simply sell the gold- and/or silver-laden charcoal for smelting rather than stripping it. That charcoal is, of course, destroyed during the process.
Leaching Operations
Over 80 operations have been identified that have experimented with leaching, were actively leaching, or were seriously planning on leaching (fig. 12). Although many of these reported activities could not be verified, table 1 presents available information on their status along with a general location, mine name, and operating company. The many new permits being granted each year indicate the great interest in leaching but make it impossible to generate a totally up-to-date list of operations.
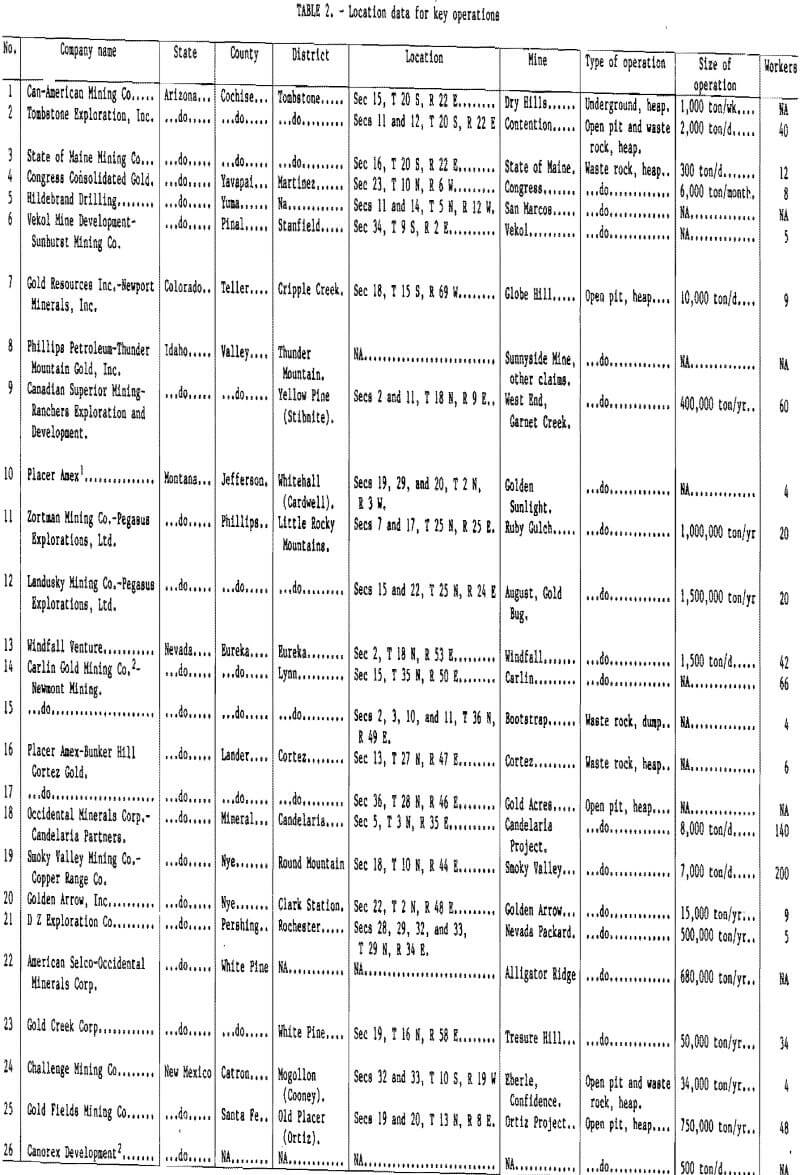
The 26 operations for which the most data were available were selected for presentation of geologic and operational parameters. Table 2 provides the location information for each, table 3 the ore characteristics and leaching data, and table 4 the extraction data. While the data contained in these tables will change, it is felt that operators or potential operators can use them to develop a feel for the range of conditions that can be expected at a particular site. The tables can also indicate possible solutions to site-specific problems. A State-by-State summary of leaching operations follows.





Arizona
Arizona has been second only to Nevada in hosting solution mining operations. The latest figures show that 14 operations had been strongly considering heap leaching (table I), and 6 of these have provided enough data to warrant inclusion in tables 2 to 4.
A heap leaching operation has been established near the Congress Mine shafts, which originally supported underground gold mining. Rock from the waste-rock dumps is being crushed and hauled to the leaching pad, where three separate heaps are being leached. After rock from the original waste piles is exhausted, the company is considering opening another underground mine adjacent to the existing shafts to provide ore for leaching.
Near Tombstone, the State of Maine Mining Co. has established an operation at an old underground silver mine of the same name. Feed for the heaps is obtained from waste rock dumps from the original operations. Plans are being made to resume underground mining to obtain new ore when the dump material is exhausted. The material will probably be crushed to boost the recovery. The State of Maine Mining Co. also manufacturers small zinc-precipitation processing plants for leaching operations.
At the nearby Contention Mine (also including the Grand Central and Flora Morrison Mines), Tombstone Exploration, Inc., is removing overburden and mining low-grade ore over the original underground working. Most of the rock can be mined by scrapers after it is ripped with bulldozers. The rock is crushed and presoaked with cyanide solution before being agglomerated into low-strength pellets. Pellets are formed by a unique inclined conveyor designed by the company. The material is then placed on a pad for leaching in 6,000-ton heaps (5 x 10 6 kg). Geologic investigations have delineated ore for at least 20 yr of operation.
Between the State of Maine Mine and the Contention Mine, Can-American Mining Co. is operating a leaching system for silver at the Dry Hills Mine. Although most of the leach material has been obtained from old mining waste rock, underground development is underway to provide new ore for the heaps. Ore is crushed to minus 3/16 in (0.5 cm) before being loaded on the heaps. The recovery averages 50 pct after 2 wk of leaching in spite of manganese contained in the ore.
Also near Tombstone, Silver Ridge Mining Co. has conducted heap leaching tests on ore from the old Nicholas and Gambasino Mines. The leaching tests were not promising because the manganese makes the silver somewhat refractory, and poor solution percolation occurred in the test heaps. Consequently ore will be processed in conventional mills.
In the late 1970’s, Minerals 71, Inc., trucked dump material from several silver mines around Tombstone to its site for heap leaching. A million-ton (0.9 x 109 kg) heap was apparently leached for 3 yr before production stopped. Since the operation shut down in February 1979, engineering data are currently unavailable.
Vekol Mine Development Co, has established a heap leaching operation adjacent to the Vekol Mine, an old underground silver mine located on the Papago Indian Reservation south of Casa Grande. Rock from mine dumps is placed on a prepared pad for leaching. This operation has been on-line since 1978. A pad with a capacity to hold 25,000 tons (23 x 10 6 kg) of ore is being prepared for heap leaching of an exposed silver ore body that crops out near the adit of the original mine. About 3 to 5 million tons (2.7 to 4.5 x 10 9 kg) of ore could be removed from this ore body by surface mining.
Near Humboldt, an experimental heap leaching pad was established at the Little Jessie Mine adjacent to its waste rock dump. The rock was placed on the heap without crushing. The grade of the return solution never reached an acceptable concentration even when it was merely recycled. Part of the problem may have been that the high clay content in the rock (due to disintegration of the schist) caused channeling. Another reason may have been that the pyrrhotite exhausted the cyanide. No further leaching activities are planned.
East of Salome an experimental operation was established on the Robinson Claims at the site of an opencut gold mine that had been located near old underground mines. Rock in and around the pit was reconstituted into a 4,000- ton (4 x 10 6 kg) heap for an experiment where leaching was carried out over several months to determine leaching parameters and to test equipment. Plans are being made to start blasting and hauling ore from the opencut for a commercial operation.
At the site of the old San Marcos gold mine, east of Wenden, a small leaching operation has been established. Dump material from the mine was placed on a small pad and leached. An experiment was carried out to determine the amenability of the ore to agglomeration. Results of the experiment look promising, and plans are being made to expand the work.
Several other operations that considered leaching but have not provided engineering data or have closed before significant engineering data could be gathered include the Montana Mine near Ruby, the King Tut Mine, the Pope Mine near Willow Beach, the Octave Mine near Yarnell, and several mines around Oatman.
California
Although much gold has been mined in California, most has been recovered from placers by dredging, hydraulic mining, sluicing, and panning, Solution mining is noticeable by its scarcity. Only a leaching operation run by Chemgold at the Picacho Mine north of Yuma, AZ, could be located. At that operation, ore is placed in successive lifts on one big pad and solution is applied by “ponding,” Gold Fields Mining Corp, has discovered a possibly leachable deposit—called the Mesquite deposit—in Imperial County, but no tests have yet been run. In addition, Anaconda has conducted agglomeration and leaching tests on tailing from the Darwin Mill, Inyo County,
Colorado
The solution mining industry is not so well developed in Colorado as it is in Arizona and Nevada; only the Gold Resources-Newport Minerals Inc, operation provided enough data for inclusion in the engineering tables.
Since about 1976, the Gold Resources- Newport Minerals Inc, joint venture has operated an open pit mine-heap leaching complex on Globe Hill. Although many of the Cripple Creek deposits are telluride (and hence not amenable to direct cyanide leaching), this mine is in an oxidated zone of the breccia from the Cripple Creek volcano. A 10,000-ton/d (9 x 10 6 kg/d) mine, supplies ore for heaps. The ore is blasted using 5-5/8-in (14-cm.) blastholes on 10-ft (3-m) centers and is leached as run-of-mine. Leaching operations extend from May to November.
Texasgulf, Inc., joined Golden Cycle Corp. in a 3-yr, $3 million exploration program to evaluate gold mining potential of Golden Cycle’s properties around Victor. One of the projects was to renovate and explore the underground Ajax Mine. The work was interesting because of the plan to try in situ leaching with chloride and iodine solutions for the telluride minerals. Since the mine follows a very steeply dipping vein structure, it was hoped that the leach solution could be applied through horizontal drill holes following the vein’s strike and collected in basins prepared in the drift after flowing down through the fracture system (fig. 13). Problems in dissolving the telluride gold ores and in collecting the pregnant solutions caused the experiment to be abandoned in 1979. The companies recently announced plans for conventional mining and milling ore from the Ajax Mine.
Dreco Mines, subsidiary of Southern Cross, Ltd., conducted a 9,000-ton (8 x 10 6 kg) heap leaching test between Del Norte and Torres in the eastern San Juan Mountains during 1981. Permits are being acquired for a 1,500-ton/d (1.4 x 10 6 kg/d) open pit mining-heap leaching operation. Another heap leaching test was conducted by E & B Mining near San Luis during the summer and fall of 1982. It is not known whether the company plans a commercial operation.
Exploration drilling for ore body delineation has been conducted at the Gold Ray Mine between Copper Mountain and Mineral Hill. Favorable ore for leaching has been discovered; this ore is oxidized into free gold, unlike the usual Cripple Creek area tellurides. A leaching plant was constructed for assembling at the site.
Yellow Gold of Cripple Creek, Inc., has adopted plans to explore the Rittenhouse Mine and refurbish various drifts and shafts. Part of the plan calls for

heap leaching the material in two mine dumps located on the property. It is not known If tests have been conducted on the material.
Recent news articles have publicized the work at Saratoga Mines’ sites near Central City. No further Information is available at this time.
Idaho
Several operations in Idaho have conducted tests on the heap leaching potential of lean ore in and around previous mining districts. Information on these tests and any full-scale activities resulting from them has been very scant; only two are discussed.
Canadian Superior Mining, Ltd., has been conducting experimental heap leaching of ores from the Stibnite area since 1974. Although the Yellow Pine Mine was originally an open pit operation for antimony and tungsten, gold was encountered at one end of the pit. Recent drilling has delineated two nearby ore bodies—the West End and the Garnet Creek—that appear favorable for leaching. In the fall of 1978, Canadian Superior conducted a 500-ton (0.45 x 10 6 kg) heap test on crushed ore; two other tests were conducted during the summer of 1979. These tests led to a leaching plan whereby the ore would be mined from two open pits, placed into 45,000-ton (41 x 10 5 kg) heaps, and leached. Production began late in 1982 after completion of an Environmental Impact Statement by the U.S. Forest Service.
Canadian Superior had also obtained the rights to explore and develop a gold mining property owned by Thunder Mountain Gold, Inc., in the Thunder Mountain district near McCall. After Canadian Superior terminated its agreement with Thunder Mountain, a new agreement was reached with Phillips Petroleum for developing the property with Coeur d’Alene Mines Corp. as the operator. The gold apparently occurs at a shallow depth that would permit open pit mining. The operation would probably be patterned after plans developed for the proposed operation in nearby Stibnite, where ore would be heap-leached and recovered by charcoal adsorption.
Montana
Heap leaching for gold and silver is rapidly emerging in Montana. Of the several operations that have expressed interest, 2 have reached full commercial production, and 1 is conducting large-scale field tests.
The two operations are on opposite sides of a peak in the Little Rocky Mountains. Landusky Mining, Inc. , and Zortman Mining, Inc., are both owned by Pegasus Gold, Ltd. One mine is a few miles north of Landusky near the old August and Gold Bug Mines. An open pit mine produces 24,000 ton/d (22 x 10 6 kg/d) to supply the heaps. Bias tholes are drilled on a 10- by 8-ft (3- by 2-m) pattern to depths of approximately 40 ft (12 m). The other mine is north of Zortman near the old Ruby Gulch Mine. Here two open pits produce 17,000 ton/d (15 x 10 6 kg/d) of ore. Blastholes are on an 8- by 8-ft (2- by 2-m) pattern. Together the two operations were estimated to contain 50 million tons of reserves at ore grades favorable for heap leaching and are annually producing 40,000 oz (1.2 x 10 5 g) of gold and 90,000 oz (2.8 x 10 6 g) of silver.
The Golden Sunlight Property 6 miles east of Whitehall hosted extensive underground mining for gold through the early 1950’s. As part of its Heavy Metals Program, the Bureau of Mines and other agencies conducted detailed economic analysis of conventional mining on the property. The present operator, Placer-Amex, first experimented with leaching on a 26,000-ton (24 x 10 6 kg) test heap. The heap was segregated into five segments (each containing a different size distribution of ore) that provided solution for five separate charcoal adsorption units. The heap was then expanded to 40,000 tons (35 x 10 6 kg) for a full production test. Feed for this effort was run-of-mine rock. High clay content in the ore caused percolation troubles; the heaps had to be periodically ripped. These troubles led to a recent decision to construct a conventional mill complex for processing the ore. Heap leaching has been dropped from immediate consideration.
In 1978 a heap leaching test was carried out on ore from the Tourmaline Queen Mine east of Boulder. Approximately 25,000 tons (23 x 10 6 kg) were satisfactorily leached. A 30,000-ton (27 x 10 6 kg) heap was constructed, and caustic was added for a new test to be conducted when the snow melted in the spring of 1979. When this test also proved successful, ore was obtained from an open pit mine for a commercial-scale heap. Since recovery from this heap was lower than anticipated, the ore was crushed and replaced on the heaps for leaching in 1981. Approximately 3 million tons (2.7 x 10 9 kg) of ore graded at 0.07 oz/ton (0.024 g/kg) gold were delineated from open pit mining.
Nevada
Nevada is currently the center of heap leaching activity. Numerous operations have been or are actively considering leaching. The Smoky Valley, the Cortez, and the several Carlin operations have all been well documented in mining literature. Enough data are available on 11 of these operations to warrant their inclusion in tables 2 to 4.
The Carlin Gold Mining Co. operation pioneered solution mining systems targeted for low-grade, disseminated, oxidized gold ore bodies. Commercial heap leaching operations began in 1971, following laboratory and pilot scale tests. About 10,000 tons (9 x 10 6 kg) of low-grade oxidized ore were leached each month between April and October. Four leaching pads were eventually placed in operation. Although the pads were generally leached about 7 days before replacing the ore, solutions were applied until their gold grade dropped below 0.015 oz/ton (0.005 g/kg). Although the ore from this mine is now processed in a conventional mill, portions of the ore body are amenable to future heap leaching. Exploration activities have uncovered two other ore deposits near the mine—the Maggie Creek and Gold Quarry Prospects—portions of which will probably be heap-leached. Contracts have been awarded to construct mining and heap leaching facilities at the Maggie Creek Deposit, and approximately 2.5 million tons (2.3 x 10 9 kg) of ore had been leached at the mine by the end of 1980.
Carlin also has been dump-leaching at the Bootstrap Mine north of its Carlin pit. The Bootstrap Mine was a surface operation from the late 1960’s until 1978. A sizable waste rock dump on the property is being leached. The dump was originally placed on a compacted ancient lakebed, which makes an impervious pad suitable for leach solution containment. The dumps are merely leveled to facilitate solution distribution.
Numerous small, underground mines around Round Mountain produced gold from the turn of the century until the late 1930’s. Now Smoky Valley has established an open pit mining and heap leaching operation near Round Mountain. By 1977, the operation was producing 85,000 oz (2.6 x 10 6 g) of dore bullion per year consisting of two-thirds of gold and one-third of silver. To achieve this rate, about 8,000 tons (7 x 10 6 kg) of ore and 7,000 tons (6 x 10 6 kg) of waste rock are mined per day. The ore is crushed, placed on a pad, and leached in one lift. The spent ore is then rinsed and moved off the pad to make room for the next batch of ore. Five pads are in continuous operation; four are being leached and one is being reconstituted at any given time. Three new pads were constructed during 1980.
Another leaching operation that is well documented is Placer-Amex’s Cortez Mine. The Cortez Mine opened as a surface operation for a conventional mill in 1969. Although the ore body was exhausted by spring of 1973, the mill continued to process ore trucked from the nearby Gold Acres Mine. During mining operations, marginal ore (below mill grade of 0.08 oz/ton [0.03 g/kg]) was stockpiled. When tests run on this material indicated that it might be suitable, a heap leaching operation began in 1971. In 1976 the conventional zinc precipitation mill circuit was converted to an activated carbon adsorption system.
Placer-Amex also operated the nearby Gold Acres leaching operation. Although currently idle, the operation has been well publicized. The site of an early mine, Gold Acres was open-pit-mined beginning in 1973 to feed the Cortez mill as a replacement for the depleted Cortez Mine ore. When this operation proved successful, a new leaching plant was constructed at the Gold Acres site. It was one of the first, if not the first, commercial applications of the charcoal adsorption system in the country. The high clay content of Gold Acres ore created lower percolation rates and recoveries than were obtained from the nearby Cortez Mine. The clayey Gold Acres ore was not suitable for leaching from lifts placed on top of each other, and periodic ripping was needed to prevent ponding. Furthermore the ore was highly carbonaceous and thus very refractive below the oxidized zone. Although approximately 5 million tons (4.5 x 10 9 kg) of ore had been heap-leached at Gold Acres and Cortez by the summer of 1977, the only current plans center around leaching waste dump rock from the idle Gold Acres property. Cortez has also announced plans to develop the nearby Horse Canyon Deposit, but it is anticipated that conventional milling rather than heap leaching will be used.
South of Eureka, a heap leaching operation has been in production several years. Near the original Windfall Mine underground workings, Idaho Mining Co. has produced ore from an open pit mine to feed its heap leaching operation. The operation is one of two that uses a ponding technique for solution distribution on the pads (fig. 8). Ore is hauled from the mine to the pad area and stacked. Berms are laid out on the surface of the pad to control the solution flow. Since the ore is a sandy dolomite, essentially clay free, percolation rates remain acceptable. Idaho Mining also opened a new pit on the property in a zone that contains some clay. It will be interesting to see if adequate percolation can be maintained on ore from the new pit. In January 1980, Windfall Venture purchased the operation from Idaho Mining.
Occidental Minerals Corp. dedicated its Candelaria operation in November 1980. At one time many underground silver mines were operating in the Candelaria district. Two of these mines—Lucky Hill and Mt. Diablo—are being stripped and open-pit mined to provide feed for a large heap leaching operation. Although the overall ore grade is good, the manganese oxide content makes it somewhat refractory. A number of experiments were carried out at the site, ranging from 500-lb (200-kg) “barrel” tests up to a pilot heap leach on 11,000 tons (10 x 10 6 kg) of ore. Based on the successful pilot test, plans were made to mine the two deposits at a rate of 8,000 tons (7 x 10 6 kg) per day along with 16,000 tons (14 x 10 6 kg) per day waste rock. Stripping began in the spring of 1980, and the first silver was poured in the fall of 1980. In 1981, its first year of production. the operation produced 1.7 million oz (53 x 10 6 g) of silver and 9,200 oz (0.28 x 10 6 g) of gold. The company preferred not to disclose engineering data from this stage of development. In June 1982, the operation closed because of the decline in silver prices. In 1983 Nerco Metals bought Occidental Minerals and re-opened the Candelaria operation.
American-Selco and Occidental Minerals combined on a successful heap leaching experiment on Alligator Ridge about 60 mi (96 km) northwest of Ely. First a 5,000- ton (4.5 x 10 6 kg) heap containing 0.18 oz/ton (0.06 g/kg) gold was leached for 2 months, with an 80-pct recovery. Next the same amount of ore was leached after the fines were agglomerated, following the Bureau’s technique (see section on ore preparation), and the same recovery was obtained in only half a month. Following these successful tests, construction began in the spring of 1980, and the operation is now producing about 60,000 oz (180,000 g) of gold per year.
D Z Exploration has been conducting experiments at the Packard Mine in an old silver mining district northeast of Love-lock for two seasons. The first experiments were on run-of-mine-sized material from the waste dumps. Since this material had weathered, enough fines were created to restrict percolation through the reconstituted heaps. Attention focused in 1979 on the Bureau’s agglomeration technique as a means to improve percolation. To test the technique on this rock, ore was crushed to three sizes—2 in (5 cm), 7/8 in (2 cm), and 9/16 in (1.4 CM)—and agglomerated using 10 lb (4.5 kg) of cement per ton (90 kg) of ore. Cement was added to the crushed ore by a unique auger arrangement. Water spray was directed at the mixture, which then tumbled down an incline to complete the agglomeration. An 8-ft (2-m) high heap containing several hundred tons of each size material was then leached. Based on the results and additional drilling, full-scale production was expected to begin in 1981, but no updated information is available.
Pinson Mining Co. opened a major open pit mining and milling complex in Humboldt County during 1981. Approximately 500,000 tons (0.45 x 10 9 kg) of material below the 0.05-oz/ton milling cutoff grade were stockpiled for future leaching. Pads were built in 1982 for several heap leaching tests involving agglomerated and unagglomerated ore, scale inhibitors, oxidizers, solution application techniques, etc. These pilot tests then formed the basis for designing a full-scale heap leaching facility that began operation in December 1982. Tests are now being conducted on material from the nearby Preble ore body.
Other operations that have been discussed by the mining press include the United Hearne Resources, Ltd., operation near Hamilton, the Getchell property recently purchased by First Mississippi Corp., the Bald Mountain property northeast of Ely, the Relief Canyon property being tested by Lacana Mining north-east of Reno, Dee Gold Mining Co.’s Boulder Creek property near Carlin, Gila Mines Corp.’s mine near Reveille, the Borealis Mine managed by Houston International Minerals Division of Tenneco, Inc., the Tuscarora operation that belongs to Tuscarora Associates, and the Gold Crown Mine in the Current Creek district operated by Great American Gold Co. The large number of leaching operations emerging in Nevada each year makes it impossible to accurately track all of them. Since this is not the purpose of the report, other Nevada operations are not discussed.
New Mexico
Solution mining operations have been relatively rare in New Mexico; only three have been identified. One of these is now inactive, while two are currently producing gold. All three are listed in the engineering data tables.
Gold Fields Mining Corp. has brought the Ortiz Mine, 35 miles (56 km) southeast of Albuquerque, back into production with a heap leaching operation. The activity stems from a mining and leaching plan submitted in 1978 to the New Mexico Health and Environment Department. Ore is being produced from a 3,000-ton/d (2.7 x 10 6 kg/d) open pit mine, which is being dewatered by a series of peripheral wells. After the ore is crushed to less than 3/8 in (9.5 mm), it is placed on a pad with a traveling gantry system. Pebble lime is added to the ore to control pH yet minimize scale problems that are normally encountered when “milk-of-lime” is added to the makeup solution. Gold recovery has averaged 78 pct with a high of 87 pct since the operation opened.
During the mid-1970’s interest revived in the old Cooney mining district around Mogollon. Mine dumps from the Confidence Mine and surface vein ore from the Eberle Mine were tested by Challenge Mining Co. for leachability with favorable results. The company has developed a 2-yr plan for heap leaching at a rate of 34,000 ton/yr (31 x 10 6 kg/yr). After 2 yr this material will be depleted, which will require underground mining in the old Eberle workings. An estimated 300,000 tons (0.27 x 10 9 kg) grading 4 oz/ton (1.4 g/kg) silver and 0.08 oz/ton (0.027 g/kg) gold remains in the mine.
In 1975, Canorex opened a 500-ton/d (450 x 10³ kg/d) open pit gold mine to provide feed for a heap leaching operation. Two adits in the ore body had proven ore reserves of 1 million tons (0.9 x 10 9 kg), grading 0.23 oz/ton (0.08 g/kg) gold and 0.63 oz/ton (0.21 g/kg) silver. A plant was constructed to handle solution from the heaps. The operation apparently went well for a while but has not produced recently.
South Dakota
Two heap leaching operations have been identified in South Dakota. One functioned at the pilot-scale level in 1980; the other has applied for the permits necessary to construct a pilot facility.
Cyprus Exploration is operating the pilot-scale leaching test at the Gilt Edge property 4 miles (6 km) southeast of Lead in the Black Hills. At the site of early mining activity, a pad and solution ponds were constructed for a 1,900-ton (1.7 x 10 6 kg) heap. Further information is unavailable at this time.
Tiaga Gold Corp.-Wharf Resources, Inc., has delineated a 5-million-ton (4.5 x 10 9 kg) ore body at 0.04 oz/ton (0.014 g/kg) gold on their Anne Creek property 4 miles (6 km) southwest of Lead. Flans are being made to heap-leach the ore with a system patterned after the Zortman-Landusky operations in Montana.
Permitting Regulations
Federal Regulations
The Resource Conservation and Recovery Act of 1976 (RCRA) has been the Federal regulatory action recently attracting the most attention from miners. Aimed at improving resource conservation and recovery through land use control, the act establishes a Federal-State permit system for hazardous waste management. Originally the mining operators were considered to be producers of hazardous waste and operators of hazardous waste treatment, storage, and disposal facilities. So many problems arose in attempting to apply the regulations to mining operations, however, that an amendment was passed in October 1980 to exempt mining from the law while studies were conducted. It is safe to assume, however, that this act will eventually impose some control. Since the material on a leaching pad and any leachate emanating from it could be considered hazardous wastes, leaching operations will fall under the purview of the act. Ground water monitoring programs will be required to assure that the uppermost aquifer will not be harmed. Although the U.S. Environmental Protection Agency (EPA) is charged with administering the permit and enforcement provisions of the act, EPA intends to pass on the permit system to the States.
EPA is also charged with implementing the Safe Drinking Water Act and, particularly interesting to in situ leaching operators, its Underground Injection Control Program. This program establishes a permitting system for five classes of wells by which fluids are injected or disposed into the ground. EPA also regulates surface discharge permits under the National Pollutant Discharge Elimination System (NPDES) and the Prevention of Significant Deterioration Program, which may affect a potential leaching system. Operators should contact the closest regional EPA office for clarification on permits required (table 5).

Leaching operations targeted for U.S. Forest Service, Bureau of Land Manage-ment, or other Federal lands will probably require an environmental assessment before permission to proceed is granted. A prospective operator should clear this through the district office of the Federal agency controlling the land. A helpful discussion of regulations and procedures is contained in “Forest Service Current Information Report No. 14,” available at Forest Service offices.
In addition, recent Bureau of Land Management (BLM) regulations (43 CFR 3800) on Surface Management of Public Lands Under U.S. Mining Laws went into effect January 1, 1981, for the purpose of preventing undue degradation and requiring reasonable reclamation of BLM lands disturbed by any mining. Depending on the level of activity and size of the disturbance, a plan must be filed and some-times approved by BLM before mining can proceed.
State Regulations
Permits required for leaching vary considerably from State to State. Table 6 lists a few key permits and gives the associated State agencies to be used as initial contacts. These contacts can provide details on which permits are required and the regulations and requirements pertaining to each.
It is interesting to note that California and Colorado have moved toward consolidating permit requirements into one office. In California, the central contact is the Department of Economic and Business Development, which provides any new business venture with a complete list of necessary permits and directions for obtaining them. The county commissions assume leadership in California during the permitting process.
Colorado has developed a voluntary process, tailored primarily for large operations, whereby a company can request a Joint Review of a proposed mining project. If a company requests this Joint Review, the State coordinates interests of all involved Federal, State, and local agencies and develops a statement of responsibilities and problems that must be resolved and a schedule of activities during the regulatory time period. Companies that do not wish to use the Joint Review process may proceed as before, by interacting with the permitting agencies, a list of which can be obtained from the Colorado Department of Natural Resources (table 6).
For the other States where leaching is practiced, table 6 lists the prime agency contacts.


Leaching Problems and Research
Percolation
Several problems hamper broader application of leaching methods for recovering gold and silver. A prime problem is the predominance of silt- and clay-size particles in some ores that prevent uniform leaching solution percolation through the ore heaps. A high clay content will cause ponding and/or channeling, which blocks much of the ore from contact with the solution. Since small particles leach faster and more thoroughly than large ones, operators frequently crush the ore before leaching. Unfortunately crushing also can produce the extremely fine sizes that promote blockage and channeling. Even without crushing, some ores have a strong tendency to disintegrate into clay through natural weathering agents and/or action of leaching solutions. Tests should be run on the ore to determine the percolation rate and metal recovery for various size distributions.
Bureau of Mines research on agglomeration techniques provides the most promising solution to clay problems; this was described in the section on Leaching Operations and in references 27 and 28. The several operators who have tried this technique have observed rather spectacular increases in percolation and recovery races. Tombstone Exploration’s Contention Mine agglomerates its ore, as do the Alligator Ridge, Packard, and Candelaria operations.
An extremely low permeability (tight) matrix presents a problem to leaching operators, again because the solution cannot reach the minerals. Weathered and oxidized ores generally leach better than unoxidized ones because oxidation breaks down an impermeable matrix. Heavier powder factors during mining may fracture the ore better, increasing the solution’s access. A fresh, tight ore may, however, yield its values only if it is crushed and ground to a small enough size to expose the metallic mineral grain. Crushing and in particular grinding, however, greatly boost the costs of preparing the ore for leaching. As previously mentioned, it is imperative that laboratory tests be conducted to determine the most economical crushing size range.
Temperature
Solution temperature is a significant factor in leaching reactions. The chemical reaction between gold and silver and cyanide proceeds much faster in warm solutions. Below 10° C (50° F) the reaction is appreciably slower than it is at normal summer operating temperatures. In practice, however, most operators work in cold weather until the solutions begin to freeze. Depending on its location, a mine can lose several months per year of potential leaching time in cold weather.
A successful research program on methods to heat solutions for leaching would provide a valuable improvement in the technology. A solar heating system would seem to be an ideal candidate since most leaching operations are located in arid regions with little cloud cover. Saline evaporation ponds may also be a possible source of heat for leaching solutions.
The only reported research on solution heating is by Smoky Valley at Its Round Mountain operation, where a 25-million- Btu/h (7.3-milllon-W) submersible kerosene heater installed in the leach solution pond has proven successful.
Solution Loss
At most leaching sites, 10 to 25 pct of the leaching solution is lost by evaporation. Since the leaching solution must contain high oxygen levels and since this is most easily achieved through spraying, evaporative losses seem inevitable. Important research topics would be to determine this loss and to measure dissolved oxygen in solutions as a function of the application method.
Besides the evaporative losses, gangue minerals in the ore consume both the cyanide and the lime or caustic soda. Consumption may run as high as 25 to 50 pct. Since the interactions between cyanide solutions and most gangue minerals are well established, no new research is recommended for cyanide leaching. For those deposits that exhibit high cyanide consumption, research on alternative lixiviants may be warranted.
Calcium Salt Scale
Calcium salts in distribution lines pose a major problem with most systems, particularly those that use lime for alkalinity control. At least one operation also experienced problems with barium salts clogging spray nozzles. The mineral scale prevents operators from using some small-orifice components in their distribution systems, such as the drip irrigation tubes used in copper leaching operations, and is particularly trouble-some in sprinklers; frequent soaking in hydrochloric acid is about the only cure. Several operators mix a scale inhibitor, such as the Surfo-H35 organic phosphate marketed by Baroid, with the leaching solution to keep the calcium minerals dissolved. Some additives, however, occasionally form colloidal suspensions that plug filtration systems.
Bagdad wigglers, first used to distribute solutions at the Bagdad copper mine, offer a possible means to combat calcium salt scale. Simple to construct (fig. 7), the large-diameter tubing will remain open much longer than the small orifices of oscillating sprinklers and is easy to free from lime scale.
Many operators have experimented with NaOH instead of lime to control pH with considerable reduction in the rate of lime scale. The relative costs must be carefully weighed, however, since NaOH is more expensive.
Research On Novel Solution Mining Methods
The Bureau of Mines has actively investigated new techniques in recovering and processing of gold and silver ores. Principal efforts have been directed toward the subeconomic, lower grade ores that are unminable and/or untreatable using traditional methods. Although major gold and silver projects have been investigated by various Bureau Research Centers, most current activity is concentrated at the Reno and Twin Cities Research Centers.
In Situ Leaching
In situ (in-place) leaching offers attractive benefits for producing metals at a minimum of capital investment and operating cost, with low environmental degradation. The technique has not been applied to gold and silver ores, primarily because of fear over public reaction to using cyanide solutions where ground water contamination is possible. Only one operation is known to have experimented with in-place gold and/or silver leaching, and that was with a noncyanide leach in an old underground mine. (See section on Colorado operations.) In this mine it was theorized that solutions could be injected into the fracture zone comprising the “vein” and that these solutions would migrate down the zone to drifts where they could be collected. To test the theory, water was injected into the vein and collected in a drift in a poured concrete trough (fig. 13). The experimenters concluded that the trough (and drift) were not wide enough to encompass the vein-fracture system; excessive solution losses occurred when the trough was bypassed. The experiment was never expanded to verify this conclusion because other metallurgical laboratory tests failed to resolve the chemistry of leaching that particular ore.
Several companies have expressed a willingness to try leaching gold and silver in-place. Successful commercial leaching operations for uranium and copper have demonstrated that in-place leaching is economically feasible and environmentally safe. If a system can be developed for in-place-leaching gold and silver that will be publicly acceptable, small and/or low-grade deposits can be mined more cheaply and easily than previously possible. The Bureau of Mines Twin Cities Research Center recently funded a contract study to evaluate the feasibility of such a system and to develop a conceptual design for it. The most likely system involved blasting via vertical holes drilled from the surface to fragment shallow ore bodies, followed by solution application from the surface. After the solution percolated down through the fragmented ore, it could be pumped to the surface from vertical recovery wells or from underground solution collection drifts. Such a system would have a very good discounted-cash-flow return on investment with payout of less than 1 yr.
Underground mines offer potential for in situ leaching. Ore would be blasted and left in stopes for leaching. After percolating through the rubblized ore, pregnant solution would be pumped to the surface for extraction of the metals.
Placer deposits offer intriguing possibilities for in-place leaching, and several years ago the Bureau conducted laboratory tests on cyanide placer deposits. At some future date it is reasonable to assume that placers could be leached by vertical injection and recovery wells like those used for uranium in roll-front deposits.
Leach Farming
A patent granted in 1973 describes a technique for leaching friable exposed ore bodies in place or conventionally mined ore that is placed in extremely shallow heaps. With this technique the ore is cultivated with standard farm equipment to loosen it to a depth of 6 to 12 in (15 to 30 cm). Leaching solution is added to the loosened ore which is periodically cultivated and kept moist for the next several days. This cultivation assures uniform contact between leaching solution and the ore and prevents downward escape of the solution.
The leached ore, which still contains the pregnant leaching solution, is pushed by bulldozer or front-end loader to a sluice box where it is slurried and pumped to a settling pond. After the spent ore has settled in the pond, the pregnant solution is pumped from the pond to a recovery plant for removing the metals. Meanwhile, the next layer of ore is being cultivated as the cycle begins again.
Although the field experiments to verify this method were not conducted on gold and silver ores, laboratory experiments on them and field experiments on other ores worked well.
Thin-Layer Leaching
Thin-layer leaching, somewhat a variant of the leach farming method, was developed for the copper mines of South America. It offers potential for leaching gold and silver ores, particularly if combined with the Bureau’s agglomeration process.
The first step in the method involves crushing the ore; minus ½ in (1.2 cm) was sufficient for the copper ores tested. Next, a concentrated leaching solution is mixed with the ore in a rotating drum until the ore contains 10 pct of solution. The ore is then placed in piles while the solution reacts with the ore. After 24 h the ore is transported to an impervious pad, where it is spread out in layers approximately 3 ft (1 m) deep. The ore is then sprayed with dilute leaching solution and/or water until the dissolved metals are rinsed out. This rinse solution is collected in ponds in the same manner as with any other heap leach and then pumped to a recovery plant for metals extraction. The damp tailing is then removed from the pad and piled in the disposal area.
Summary
Gold and silver leaching operations have blossomed in many mining districts of the Western United States. Most are associated with old “vein” mines where the elements were deposited in fractures near volcanic or other igneous activity. A few large operations have also been established on oxidised sedimentary deposits containing very fine, disseminated gold.
In surveying current operations over 80 were found that had conducted tests or were actively leaching on a commercial scale. Sufficient engineering data were obtained for 26 of these operations to justify their inclusion in tables that cover location, geology, heap configuration, influent solutions, effluent solutions, and extraction.
One of the first problems encountered by a hopeful leaching operator is obtaining the necessary permits from Federal, State, and frequently local agencies. To provide a start, key Federal and State agencies are identified in tables 5 and 6.
The main problems hampering leaching operations are unfavorable mineralogy and poor solution percolation owing to high clay content. Cold temperature effects on gold and silver solubility and rapid lime buildup in the distribution system also severely hamper the operations. Recent and ongoing Bureau research and continued work at various mining operations are helping improve the techniques.
