Table of Contents
- Introduction to Gold and Silver Leaching
- General Precious Metal Leaching Theory
- How Density of Solutions Affects Leaching
- What is the Effect of Aeration on Gold and Silver Leaching
- Oxidizing Agents
- Decomposition of Reagents
- How Alkalinity (pH) Impacts Silver and Gold Leaching
- Effect Grinding and Particles Size
- Particle Size Classification
- Gravity Gold Recovery in Grinding Circuit
- Pre-Leaching Pulp Thickening
- How does Agitation Affect Gold and Silver Leaching Kinetics
- Gold and Silver Precipitation by Zinc Powder
- Screen Analysis — Microns
- Comparing Flotation and Cyanidation VS Whole Ore Cyanidation
Introduction to Gold and Silver Leaching
The cyanide leaching process is the most important method ever developed for extracting gold from its ores. The early development of the process is attributed to a Scotchman, John Stewart Mac Arthur, in collaboration with the Forrest brothers. The method was introduced into South Africa in 1890. From there it spread to Australia, Mexico and the United States. Now it is used in practically all the major gold mining camps of the world.
The reasons for its widespread acceptance are economic as well as metallurgical. It usually obtains a higher recovery of gold than plate amalgamation and is easier to operate than the chlorine or bromine process. It produces the final product in the form of practically pure metal. Thus the production from a large cyanide mill will be represented by a comparatively small gold bar, which is easy to transport. Accordingly gold mines can be located in relatively inaccessible districts served only by aeroplane or mule train.
However, the gold metallurgist must be familiar with the other processes of gold treatment, such as amalgamation and flotation, as they are frequently used as an auxiliary to the cyanide process.
General Precious Metal Leaching Theory
Before going into the theory of the cyanide process, a brief review of the chemical properties of gold may be beneficial.
Gold does not oxide (tarnish) at ordinary temperatures nor is it soluble in sulphuric, nitric or hydrochloric acids. It does dissolve in aqua regia (a mixture of nitric and hydrochloric acid) also in some chlorine and bromine compounds. On the latter reaction was based the bromo-cyanide method used on some refractory ores in the early days of gold mining in Australia. Gold is soluble in mercury, uniting with it to form amalgam. However, the main chemical property of commercial interest is that gold is soluble in dilute cyanide solutions.
The basis of the cyanide process is that weak solutions of sodium or potassium cyanide have a preferential dissolving action on small particles of metallic gold and silver over other materials usually found in gold ores. However, there are a few minerals known as cyanicides that have deleterious effects which are discussed later.
Cyanide is the general descriptive term applied usually to sodium cyanide, NaCN. However, the early work in cyanidation was based on the use of potassium cyanide and strengths of solution as well as basic formulae are still in terms of that chemical. It is to be noted that the cyanogen radical (CN) actually has the dissolving power, the alkaline base of potassium calcium or sodium merely giving a chemical stability to the compound.
The main difference between the alkaline cyanides, aside from their cost, is their relative dissolving power. This depends entirely on the percentage of cyanogen radical present.
Elsner’s equation is generally accepted as expressing the action of gold in dilute cyanide solutions; 4 Au + 8 KCN + O2 + 2 H2O = 4 KAu (CN)2+ 4 KOH. Thus, when fresh surfaces of gold are exposed to the action of cyanide in an aqueous solution containing free oxygen, a gold cyanide compound will be formed and a hydroxide (alkaline).
Strength of solution is usually about one pound of cyanide (KCN equivalent) to a ton solution (water). This is usually sufficiently strong for most straight cyanide circuits and experimental work has shown that maximum dissolving power is obtained at this strength. Furthermore, a weak solution is less affected by cyanides, and danger of poisoning from fumes formed by evaporation in hot weather is decreased.
Gold sulphide concentrates, obtained by table concentration or flotation, are frequently treated by higher strength solutions. These concentrates usually require very thorough study, as outlined later.
Strength of solutions is usually expressed in pounds of equivalent potassium cyanide per ton of solution. 1 lb. cyanide to 1 ton of water = 0.05% solution; 2 lb., 10%, etc.
Temperature of solution is also important in maintaining efficient dissolving action. Especially in cold climates, the solutions are frequently heated to about 70°F. Above this temperature the loss of cyanide by decomposition becomes a serious factor. Theoretically, gold dissolves fastest in a solution at a temperature of 138°F.
How Density of Solutions Affects Leaching
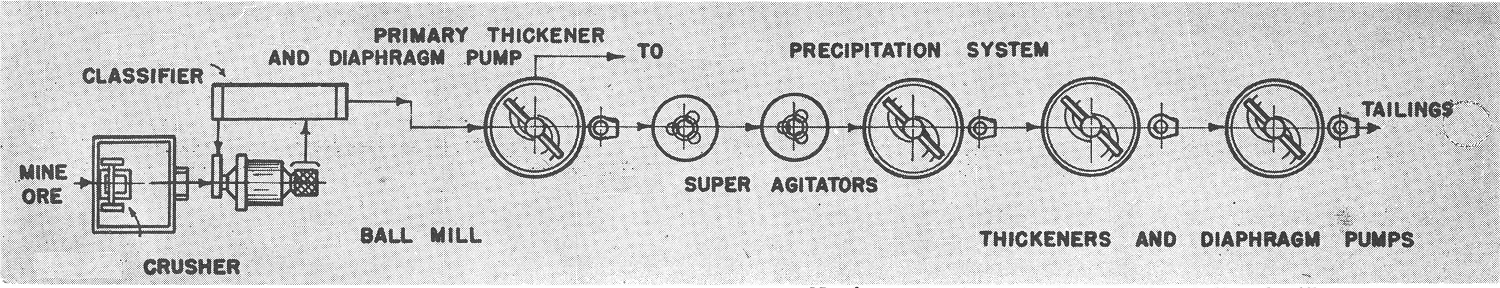
To maintain maximum capacity and minimum loss of valuable material in solution, it is usually advisable to maintain the utmost densities in the mill circuits. It should be kept in mind that for every ton of water added to the mill circuit, a ton of water must be removed to maintain equilibrium. This discharged solution not only contains reagents, such as lime and cyanide, but also dissolved gold, even if only in minute quantities.
The higher the density of the feed to the agitator the greater the capacity of the agitator, or conversely, smaller or fewer agitators are required. Assuming an ore where the solids have a specific gravity of 2.6, one ton of solids as 30% solids (70% solution) will occupy 86.7 cubic feet, while at 50% solids will only occupy about half that space, namely, 44.31 cubic feet. Also there is apt to be more settling of sand fractions which may cause mechanical difficulties when treating a dilute pulp. Accordingly agitator densities are usually kept from 30% to 60% solids, Grinding capacity in a ball mill is also limited if the density drops below 70% solids.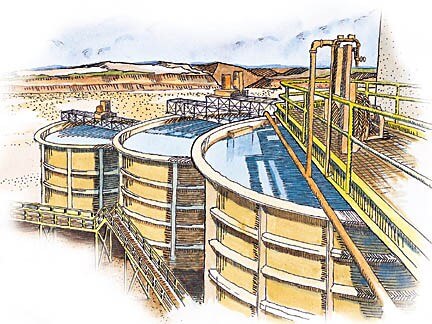
What is the Effect of Aeration on Gold and Silver Leaching
Another of the prime requisites of successful cyanidation is free oxygen. Pure oxygen is too expensive to use, instead atmospheric air is the customary source of the required oxygen gas. Some interesting experiments have been conducted using Ozone but the practice has not been adopted commercially on account of the cost in the gold leaching process.
For efficient dissolving, it is necessary that the air come in actual physical contact with the gold particles. As these particles are usually very sparsely distributed through the pulp, it means that the air bubbles should be thoroughly dispersed and a huge excess be used beyond theoretical requirements of air. Under the subject of Side Air-lift Agitators will be found required air volumes.
Oxidizing Agents
These oxidizers may be sodium peroxide, potassium permanganate or manganese dioxide. They act in two ways—by a nascent or active condition and so accelerating the dissolving of gold, and by oxidizing deleterious impurities that may be present in the ore or solution.
It has been found in some mills that due to increases in tonnages or changes in the ore, that extra aeration is necessary. Various methods have been used for this, one of which is reported, of placing a ring of air jets around the circumference of each of the agitators. In this particular instance, the agitators were 18’x 21′ and eight of these jets were placed on each ring. The jets consist of ¼ pipe, running from a 1″ header, equally spaced around the circumference of the agitator tank and projecting 10′ under the surface of the pulp. High pressure air from the mine compressors was used. Violent aeration and agitation resulted, in addition to regular air-lift agitation of these machines.
Another method is based on the dispersal of the flow of pulp as it enters the various tanks in one large thick stream. At this property trays were built of 1/16″ steel plate about 4″ high and 2′ square. To the bottom of these trays was welded ¼” mesh steel screen plate. These baskets or screens were hung about 1′ under each discharge launder, or pipe, and the flow of the pulp stream spread to cover as much of the screen as possible. In this way a single stream of pulp was converted into many streams. On one side of the screen, an inch or so below, a 1″ pipe connected with the main compressed air line is anchored. Small holes about 1″ apart are punctured along its length facing the flow of pulp. When the air is turned on, the many streams of pulp passing through the screen are thrown across the tank in very small particles. The pulp in this way is much better aerated than under the former method.
Another method noted in the field is the insertion through the sides of the tank, well below the top of the pulp, of 3/4″ pipe, the ends of which have rubber hose about 6″ long securely wired to them. The end of this rubber hose in turn is fastened by a wooden plug securely wired in place. Then a fine slit is cut lengthwise along the bottom of the rubber hose with a penknife. When the compressed air is turned on, the air pressure is sufficient to open this slit and allow the air to bubble into the agitator. However, if for any reason the air pressure drops, the rubber slit automatically closes on the reduction of the pressure and no pulp enters the air piping.
Decomposition of Reagents
As has been previously outlined, the amount of reagents actually required for dissolving the gold is extremely small. However, frequently the amount of reagents used is much higher and certain causes should be recognized and, if possible, remedied. These may be briefly listed as follows:
- Impure Water
- Cyanicides
- Mechanical losses
The source of water is very important, not only from the viewpoint of the quantity available at all times, but also the quality. In some districts the only water available is from small lakes or ponds and, as such, is frequently contaminated with organic material and soluble salts. This water may be highly reducing in its action. Extra lime treatment may be necessary before this water joins the mill return stream. Lead nitrate solutions may be added to aid in the precipitation of the soluble salts. Chemical oxidizers such as potassium permanganate are also used. Some of these problems are also discussed further under the heading of “Clarification.”
Certain materials known as cyanicides may be present in the ore. A cyanicide may be defined as a natural occurring material that destroys cyanide. Pyrrhotite is one of the best known. It combines with the cyanide giving ferro-cyanide and sulphocyanide. It is stated that stibnite requires extremely low alkalinity to prevent its solubility in solution. The reverse is true in the case of sphalerite, where high lime tends to reduce zinc solubility.
Ores often contain copper, antimony, arsenic, cobalt or nickel sulphides which go into solution through the action of the cyanide.
Although the dissolving rate of these materials may be controlled to some extent, the solutions in time will lose their potency due to being recycled. Then it is necessary to bleed off part of the fouled solution and restore the balance by the addition of fresh stock. After the solutions have been de-aerated and precipitated, it is also necessary to positively aerate them before being again used. This aeration is frequently accomplished by allowing the stream of solution to fall vertically several feet into an open solution tank. This aeration not only restores the free oxygen to the solution, but also partially regenerates some of the combined cyanide.
Mechanical losses occur in two ways:
(a) Accidental losses.
(b) Inherent losses.
The first is due to spills and leaks on account of poor launder design and tank spillages. Losses also occur when it is necessary to dump agitators, classifiers or thickener tanks, due to power failures or mechanical difficulties.
Inherent losses may also be considered from two view-points, namely, those occurring only in a new circuit, and those occurring continuously. The first is due to solutions soaking into the fresh wood tanks and may occur over a period of two or three months. The second is due to losses from filter discharges, etc. Naturally it is desired to keep these losses at a minimum, as they are a flat charge against the cost of operation. For example, a filter cake may have 10 to 12% moisture in it. A heavy water wash on the filter will reduce the amount of chemicals in this moisture, while re-pulping and second filtration may be advisable in some cases. A cost analysis in every case is desirable.
How Alkalinity (pH) Impacts Silver and Gold Leaching
To reduce the amount of cyanide destroyed, lime is added to the solution to maintain a “protective alkalinity.” It is usual to keep this alkalinity at from ½ to 1½ lbs. per ton of solution. Lime has a further beneficial effect of hastening settlement of finely ground rock, or slimes, in thickeners, and it further precipitates certain undesirable substances.
In order that the lime will begin its protective action as soon as possible, it is usually added with the fresh ore in the ball mill. It may be added dry or as a milk of lime. Frequent and systematic sampling of mill solutions at various predetermined points in the dissolving circuit is advisable. Then the operator can control the lime and cyanide strength and be certain at all times that minimum required strength is being maintained. Cyanide is usually added in the freshly aerated solution pumped to the grinding circuit, although sometimes blocks of cyanide may be suspended in baskets in a dissolving circuit to remedy some local trouble.
Effect Grinding and Particles Size
Attention will now be given to the theory of the various mechanical stages. Of these the most important from the cost viewpoint is grinding, which may account for 40 to 70% of total process cost.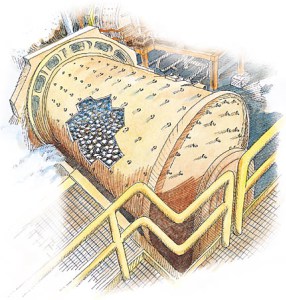
Grinding is usually performed in a ball mill for the purpose of reducing the ore to sufficient fineness that the gold particles may be exposed to the dissolving action of the cyanide solution. This dissolving action may begin either in the ball mill or in the agitators. In the former case, the grinding is done in cyanide solution. If metallurgically possible, this is highly advisable, as 30% to 85% of the gold is usually dissolved here, thus greatly relieving the metallurgical load on the agitators. The dissolving conditions in the grinding circuit are ideal, as the fresh metallic faces, as exposed, are instantly brought in contact with the cyanide solution, which usually is at a comparatively high temperature.
However, some ores contain cyanicides that necessitate pre-liming, that is, grinding in alkaline water.
These solutions are then thickened to remove this deleterious material before the cyanide is added. This is often necessary when treating concentrates.
As a general rule, the finer the state of division of the gold, the finer the grinding required. This particularly applies to ores where the gold is intimately associated with the sulphides. It is usually considered also that the finer the grind, the higher the percentage of extraction. However, it is necessary to maintain an economic balance inasmuch as the cost of grinding greatly increases with the fineness of grind, and frequently the ore becomes harder to grind at finer meshes. Over-grinding may not only result in very fine slimes which are hard to handle in thickeners, but may also result in coating the gold particles with foreign matter hammered into them, by the falling action of grinding balls.
In some ores a large percentage of the gold occurs with the sulphides, which may only constitute a small proportion of the ore. Extremely fine grinding may be necessary to liberate this gold from the enclosing sulphides. In these cases, if the entire mill feed was entirely ground, the cost of the operation would be very high. It is usually advisable to remove sulphides from the primary grinding circuit and give them a separate grinding treatment.
The Mineral Jig is being widely used to do this work. This machine may be operated with a continuous discharge, if desired, to feed the sulphides to a small regrind unit. In closed circuit with this secondary circuit may be installed Super-Agitators to aid the dissolving of the gold in this refractory material. These super-agitators were especially developed to give the intense agitation and aeration necessary on this type of material. Tailing from this unit then joins the main cyanide circuit. Flotation cannot be used, as cyanide is a strong depressant for sulphides.
By using a secondary grinding circuit as outlined above, the main classifier overflow may be comparatively coarse, as it contains mainly gangue material. Any fine gold attached to the clean quartz particles would quickly be dissolved, and thus the length of contact time in cyanide may be at a minimum. The gold in the sulphides, which requires fine grinding, is given a separate intensive treatment. However, as this separate portion represents only a small percentage of the mill feed, the cost of installation and operation is materially reduced over that required if the complete tonnage were given this thorough treatment.
Grinding in ball mills is done wet because of the higher efficiency of wet over dry grinding, and of the dissolving effect that is available in the solution.
Control of size of finished particle is maintained by classifier of either the rake or spiral type. The fineness of grind is usually specified by the percentage of finished material that will pass through a standard screen. For example, 70% minus 200 mesh. The capacity of the ball mill is usually considered to vary as the 2.83 power at the diameter and directly as the length. As the effective diameter of the ball mill is measured inside the liners, it is important to know exactly whether the diameter of the ball mill in question is measured inside the shell or inside the liners when calculating capacity.
Particle Size Classification
The purpose of classification is to control the size of the material being fed to the dissolving or agitation circuit. The discharge from the ball mill flows into the classifier where it is split into a sand and slime portion; the sand particles being returned by mechanical means to the feed end of the ball mill, while the material of predetermined fineness overflows the discharge end of the classifier and is ready for the agitation circuit. This step is termed “grinding in closed circuit” and greatly increases the efficiency of the entire grinding circuit.
This is due to the fact that the particles as ground sufficiently fine are removed from the pulp by the classifier and sent to subsequent treatment while the coarse particles are returned for further grinding. Thus the power consumed by the ball mill, which is a major factor in any milling circuit, is kept at a minimum.
Gravity Gold Recovery in Grinding Circuit
For efficient operation of the cyanide circuit, it is necessary to remove the coarse gold from the grinding circuit as soon as it is released, otherwise this gold will become lodged behind the liners of the ball mill and not be recovered until the ball mill is relined. It may also be lodged in the classifier and thickener tanks. Especially in a small high- grade mill, this may be a serious tie-up of gold. Furthermore, coarse gold particles are slow to dissolve in cyanide solution, and accordingly there is a possibility that they will be discharged in the mill tailing before being completely dissolved. A further advantage is that this removal increases the capacity of the grinding circuit. This removal is either done by blankets, traps, concentrating tables or the Mineral Jig.
In the Little Long Lac district of Ontario, Canada, five of the mills are cyanide mills and five are flotation mills. Of the former, three grind in cyanide solution and two in water before cyanidation. All ten mills have either blankets, strakes, jigs, unit cells or combinations of these in their primary grinding circuit. Six employ amalgamation to recover a proportion of the gold. The following table shows the methods used in concentrating the gold in the grinding circuit at several mills and percentage of gold reported as recovered by these means:
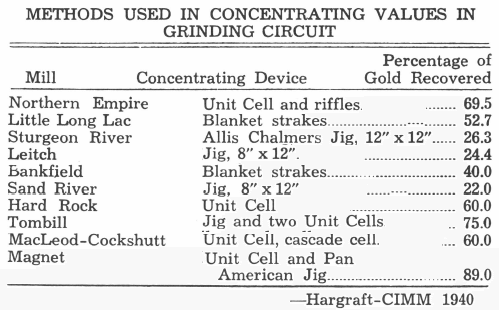
A further benefit is that this reduces the size of the mill building and in cold climates, the cost of heating is a major item. Furthermore, the mineral jig acts as a safety valve for the cyanide section. It enables the use of smaller agitators. Lower grade solution is going to the thickeners and so the final soluble loss is lowered. It further has the effect of reducing the amount of solution that is handled by the precipitation system, thus lowering the cost of this unit and the amount of chemicals involved.
Pre-Leaching Pulp Thickening
Thickening may be defined as the mechanical process whereby excess solution is removed from a pulp. Frequently the classifier overflow contains more water than is desired in the succeeding agitations units. Therefore, the excess rich solution is removed by thickening and sent to precipitation.
To determine the capacity required, it is necessary to conduct settling tests but certain main factors may be considered in operation of the thickener.
- The greater the specific gravity, the more rapid the settling rate.
- With the same specific gravity, larger particles will settle faster than smaller particles.
- Round or cubicle shaped grains will settle more rapidly than irregular shaped particles.
- With an increase in temperature, the viscosity of the solution is decreased and, therefore, the settling rate increases.
- Lime frequently acts as a coagulant to improve the settling rate of fine material.
- Area of the thickener is the main function of its capacity.
- The depth of the thickener tank influences the capacity depending on the dilution of feed and the dilution of the underflow.
- Slime will settle to a maximum density beyond which it will not compress further.
Most thickeners in cyanide circuits are operated so that the slime level will be from 6″ to 1′ below the overflow. In this way a clear solution is obtained from the overflow launder. This slime level is carefully watched and measured during the shift, due records being kept. If it should rise, it is necessary that either the tonnage be reduced or certain coagulants such as additional lime be added to further flocculate the slimes. Starch solution is sometimes used for this purpose also but is more generally considered to aid in the clarification process.
How does Agitation Affect Gold and Silver Leaching Kinetics
Agitation may be considered as the mechanical method of mixing the pulp with an excess of air in circular tanks of sufficient capacity to allow the balance of the gold to dissolve in the cyanide solution. These agitators are of various types of construction, being divided basically into two types, namely, those depending entirely on airlifts and secondly, those depending on a combination of air and mechanical stirring. The first is best known as the Brown or Pachuca tank in which the height is at least three times the diameter. It depends entirely for its stirring action on a column of air rising from the central bottom cone.
The mechanical agitators use an excess of air in the side or centre airlifts for elevating and aerating the pulp while depending on mechanical stirring devices at the bottom to help keep the pulp suspended.
To prevent short-circuiting of material, it is advisable that at least two agitators be used in series, and preferably three, instead of one large agitator. With some refractory ores, it is also advisable to consider inserting an extra thickener in the agitation circuit so that the strong cyanide solution may be removed and fresh added to aid in the slow dissolving. The efficiency of an agitator is also dependent on the method of introduction of the air, as finely dispersed air bubbles are necessary for a fast dissolving action. The pulp dilution is kept to a minimum to reduce the size of agitators necessary and to prevent undue settlement of the sands.
Clarification of Gold Pregnant Solution
After the pregnant gold-bearing solution is removed from the thickeners and before it is sent to precipitation, it is necessary that the impurities and suspended solids be removed. This is done by the process of clarification and it is on the efficiency of this step that the entire precipitation cycle depends.
Various types of equipment are available for this work. However, the basic principle of most of them is essentially the same and consists in sucking the solutions through a canvas or other coarse filtering medium which is suspended by means of a frame in the solution tank. This filtering medium is frequently coated with inorganic material such as diatomeceous earth to assist in the filtering process and to aid in the removal of the fine slime.
It is absolutely essential that the solutions after clarification be absolutely clear. It is also essential that precipitation take place immediately after clarification and de-aeration, otherwise there is danger of contamination of the solution. Besides the lime that is added to the thickeners to aid in the coagulation of the slime, it is sometimes necessary to take additional steps to obtain efficient clarification.
For example, reference is made in the February, 1936 issue of Mining and Metallurgy of the use of caustic starch at the Dome Mill in Northern Ontario as introduced by Mr. C. B. Dowsett. At that mill the grinding is done in water and the solutions pre-aerated before cyaniding. Lime is added during the pre-aeration. Trouble occurred in the large silica content in gold precipitate. This was caused either by the zinc precipitating the silicia from solution or else acting as a coagulant for dispersed silicia gel. By the addition of a compound of starch and caustic soda, the slime settlement was greatly improved and the precipitation was run satisfactorily for ten to fifteen days instead of from the three to five days as formerly. The quantities used at the Dome are 16 pounds of starch and 8 pounds of caustic soda for each 1500 tons treated each day.
The caustic starch is prepared as follows:
To 40 parts of water kept boiling in a drum by the use of steam, is slowly added one part of starch made into a slurry with four parts of cold water. The mixture is then boiled for ten minutes, after which is added half a part of caustic soda dissolved in water. After boiling another ten minutes, the solution is diluted to a convenient degree to permit accurate metering into the pulp flowing into the thickeners. The reagents should be added sparingly at first for in some instances increased flocculation in thickeners may adversely affect concentration, especially where very fine grinding is demanded.
Reference is also made in a recent issue of the Mining Magazine (London) to the use of starch in the thickeners of the Raub Australian Gold Mining Company. At that property, trouble was experienced in thickening of the flotation concentrate and this was remedied by the use of a starch addition. To make the caustic starch employed, five gallons of water were added to 40 pounds of tapioca flour to form a lump-free slurry and this was slowly stirred into 35 gallons of boiling water, stirring continuously until the proper solution had been effected. This is then causticized with 1.2 pounds of sodium hydrate in solution and stored for use. About two pounds of this caustic starch were added to the thickener for each ton of concentrate.
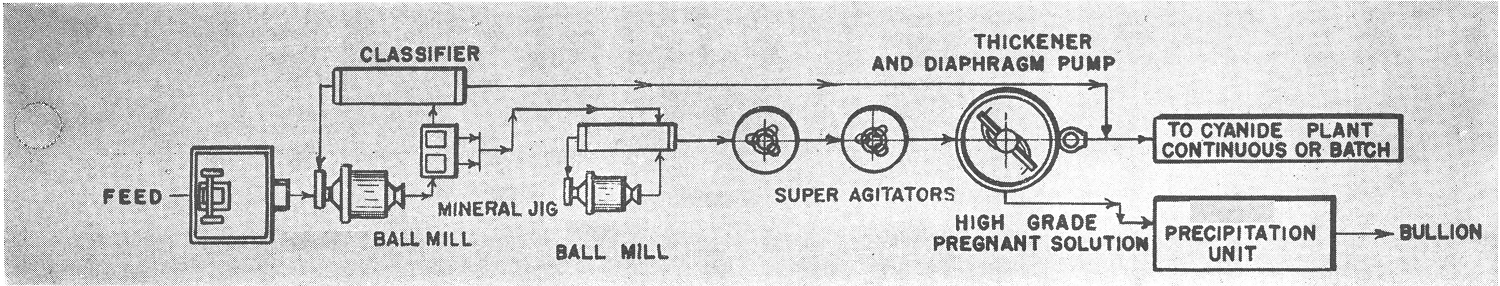
Gold and Silver Precipitation by Zinc Powder
After the solution has been clarified and immediately preceding precipitation, it is necessary to remove the dissolved oxygen from the solution. This is done by a de-aeration process developed and marketed as the Merrill-Crowe Process. For further information on this process, reference is made to that company’s bulletins.
The gold is then removed from the solution by precipitation with zinc dust. Dust is used instead of the zinc shavings formerly employed on account of the large surface area made available. The method is based on the fact that gold and silver are electronegative to zinc and that the following reaction occurs in the precipitation process.
KAu (CN)2 + 2KCN + Au + H2O = K2Zn (CN)4 + Au + H + KOH
The following reaction may also occur which will account to some extent for the excess of zinc consumption over theoretical needs:
Zn + 4KCN + 2H2O = K2Zn (CN)4 + 2KOH + H2
Soluble lead salts such as lead acetate or lead nitrate are sometimes added to cyanide solutions to form with the zinc a zinc-lead couple of greater activity as a precipitant than zinc alone. Frequently a drip of strong cyanide solution is also added to the zinc mixing cone feeding material to the solution.
In case difficulties are experienced in the precipitation process, it is advisable to thoroughly check the clarification and also the airlines leading to the de-aeration process. An air leak in this latter operation may have serious affect on precipitation.
Screen Analysis — Microns
To determine the finest fractions of a gold pulp it is necessary to use other mechanisms than the conventional laboratory screen. The Haultain infrasizer used with the Haultain Superpanner is most widely used in Canada for this purpose. Screen analyses are specified in micron (one mm =108 microns). A 200 mesh Tyler Screen has an opening of 0.074 mm. = 75 microns. Finer relationships are:

Comparing Flotation and Cyanidation VS Whole Ore Cyanidation
A thorough study of this subject is contained in an interesting article by J. P. Dick entitled “Mining and Metallurgy at Moneta Porcupine” in the March 1941 issue of the Canadian Mining and Metallurgical Bulletin. The following comparison is given:
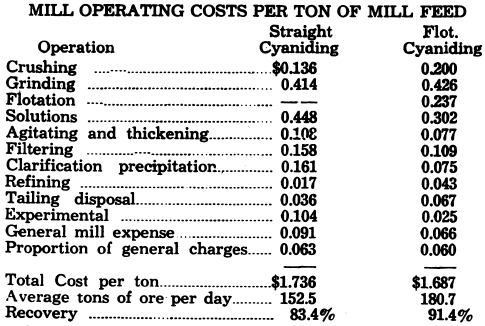
Increase in cost of crushing and grinding is due to finer ball mill feed and regrinding flotation concentrate before cyaniding to 64%—10 microns.
To supplement these figures it should be considered that where metallurgically possible, lower cost per ton will be produced from a combination circuit. However this should not be accepted blindly, as the author believes that below 100 tons a day straight cyanide circuit will be cheaper to operate.
A further point to consider is that frequently the ore at the surface of a property is comparatively easy to treat, and a straight cyanide circuit is naturally installed, usually with a mineral jig in the grinding circuit to remove free gold. As further development is done in depth the character of the ore frequently changes to include primary gold- bearing sulfides. Sufficient tonnage has also been blocked out to warrant mill expansion. Then the logical action is to install flotation cells to remove the sulfides, which are reground and cyanided in the original cyanide mill. Only a new grinding circuit, in addition to the flotation machine, is needed to increase the mill capacity. The ultimate increase will depend on the ratio of concentration obtainable by flotation with a flotation tailing that can be economically discarded. This ratio and consequent increase may range from 2:1 up to 35:1.
Even if the flotation tailing cannot be economically discarded, as such, these are certain additional considerations. For example: one concentrator with a very low record of cost feeds the flotation tailing to a hydroclassifier. The slime overflow is discarded. The sand fraction is given an agitation of about four hours, resulting in small agitators, and on account of the fast settling characteristics of the sand, thickeners with a capacity of about 0.3 sq. ft. per ton are successfully operated. Accordingly a very small, compact, cyanide mill was installed.
Batch Cyanidation
The batch process of cyanidation is usually applied in two different types of mills:
1. Where the amount of material to be treated is quite small.
2. Where the material to be treated is quite erratic in nature.
The first type is exemplified by Guysborough Mines Ltd. The flowsheet for which is shown here.
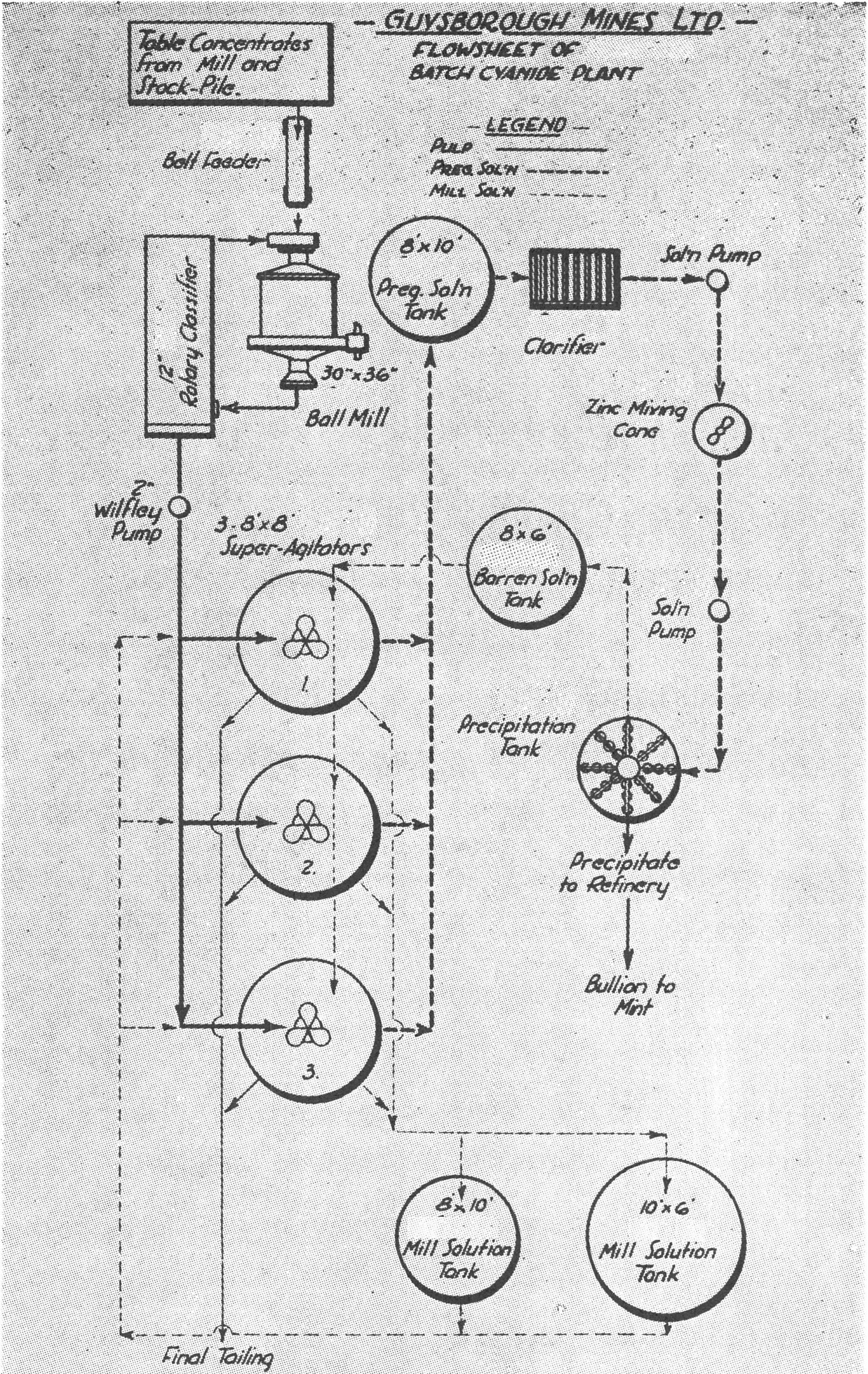
At some properties the type of material being handled is quite erratic and so to obtain the maximum extraction the batch method is used. The material is agitated, with perhaps several solution changes, until final assay of residue is satisfactory. Then the material is discharged from the tanks.
Super agitators with float decantation devices and bottom discharges are ideal for this type of work.
Cyanidation—Sand Leaching
Canadian and American cyanide practice usually results in grinding all the ore to a uniform fineness—“all sliming.” This is made possible by modern crushing and grinding equipment, and by modern continuous thickeners and agitators. This practice overlooks many of the advantages of the sand leaching systems that are being successfully operated in other parts of the world.
Especially on low grade gold ores the use of sand leaching should be carefully considered. It has an advantage of allowing a coarse grind and a comparatively simple plant. The main requirement is, of course, that the major part of the gold, can be dissolved at the coarse grind.
In practice, efficient classification is absolutely essential for the successful operation of a leaching plant. Even a small percentage of slimes will seriously reduce or even destroy the porosity of the sand bed. Size of particle in the feed is of little concern as long as granular.
Accordingly the discharge from the grinding circuit is classified, the overflow being either treated separately in a slime circuit or discarded, according to its value. The sand is pumped to one of a series of large diameter shallow tanks with porous false bottoms. The sand is evenly distributed in the tank by means of a mechanical distributor. Cyanide solutions may be introduced from the bottom and allowed to percolate upwards, or fed from the top and allowed to seep downwards. Usually the strongest cyanide solution, with the required dissolved lime, is added first, followed by weaker solutions and then one or more water washes. Between each percolation the sands should be allowed to drain so that air will reach the gold particles being dissolved.
After the percolation has been completed, the sand is usually discharged through doors in the bottom of the tank onto a conveyor belt that removes it to the tailing pile. Some operators prefer final washing in a series of washing classifiers after cyanidation in the tanks has been completed.
The number of percolation tanks required depends on the tonnage capacity of each tank, the daily tonnage, and the total time required for a cycle of operations. Leaching and washing may require from two to ten days depending on the ore.
Gold Leaching Flowsheet C1
This flowsheet is for a medium grade ore where a high recovery is made by the Mineral Jig in the grinding circuit and where a large percentage of the fine gold is dissolved during grinding. The solution is then sufficiently high grade to warrant removal by the primary thickener and sending to precipitation. Fresh aerated solution is then added to the agitators where the remainder of the gold should be dissolved.
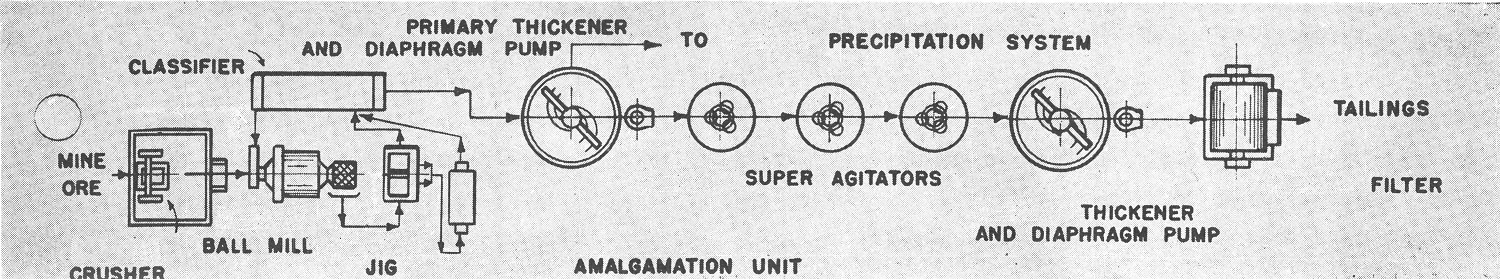
Three agitators are shown. A multiple number is necessary to minimize the possibilities of short circuiting of pulp. This is especially important when treating high grade pulp.
Secondary thickening is sometimes omitted, with the agitator pulp going direct to filtration. This can be done with low grade pulp, the agitators being operated at a high density. However in most cases the secondary thickener is advisable as it stabilizes the load to the filter, both in tonnage and density, allows flexible operations of the preceding agitators and decreased soluble loss by reducing the soluble feed to the filter.
Two stages of filtration are also used in some mills. A repulper may be used to run fresh water with the discharged cake from the primary filter or a mechanical agitator may also be inserted to insure thorough mixing.
Solution flows will depend on the type of ore being treated, and, frequently the personal ideas of the mill operator. Ample storage capacity without fluctuations of head is necessary.
Continuous Counter-Current Decantation
The CCD system of washing cyanide pulps is the logical development of the introduction of the continuous slime thickener and the batch method of cyanide decantation. In operation, a series of thickeners is used. Pulp is fed into one end of the series, water into the other end.
The flow of pulp and water is thus in the opposite directions. Accordingly the pulp becomes progressively lower in soluble content as it passes to the discharge. Conversely, the water added at the discharge end passes forward, increasing in strength in lime, cyanide, and dissolved gold.
The CCD system is used to take the place of, or supplement, filtration. Soluble loss is usually higher than filtration with heavy water wash. Space required in a mill is greater. But operating costs are usually lower.
It is possible to calculate the soluble losses in a CCD system by a series of simultaneous equations. For the details of the mathematics concerned refer to E. M. Hamilton’s “Manual of Cyanidation.”
Precipitation by Charcoal
Taking advantage of the natural tendency of charcoal to absorb gold dissolved in cyanide solution, the Chapman process (U. S. Patent 2,147,009) may have applications to unusual types of gold ores. It is not claimed that this method can compete with present cyanide methods when the ore to be treated is easily cyanided. However it has interesting possibilities for future consideration.
The ball mill, in closed circuit with a classifier, is fed with ore, cyanide, lime, and carbon as charcoal. The classifier overflow is thickened and then agitated. The gold is dissolved by the cyanide solution and then its soluable gold-cyanide compound is absorbed by the finely divided charcoal. Flotation then recovers the charcoal.
In the application of the Chapman process to a high grade gold ore, tailing from the primary flotation circuit is again floated in a scavenger circuit, the low grade concentrate from which is fed to the grinding circuit.
Chapman states that the double-stage process is not as complicated as might appear: for low grade tailing satisfactory absorptions have been obtained with two lb. of charcoal per ton, and three to seven lbs. for ore assaying up to 0.4 oz. gold per ton using the double-stages process. Thick pulps give better results than thin pulps. Silver is not as effectively treated as gold.
It is estimated that the capacity of a flotation machine floating the charcoal would be about three times that treating sulfides. Incidentally if the ore contains sulfides it may happen that these can be concentrated with the carbon.
The charcoal concentrate is filtered, dried, and shipped to a smelter. Or it may be dried, burned, briquetted and then shipped to a smelter.
Absorption of gold in various tests have ranged from 65% to 85% with recovery of the carbon from 90% to 99%.
Another process using the charcoal as an absorbent was that developed by Crawford (U. S. patent 2,186,799) for treating low grade ores. In this process the charcoal may be added to the cyanide circuit any place. Theoretically charcoal formed at 800 °C. is best for this work. Actually carbon burned from a variety of woods at different temperatures seems to work satisfactorily.
Treatment of Graphitic Gold Ores
Carbon acts as a precipitant of gold and thus when occurring in an ore as graphite will cause premature precipitation. At some properties it has been found that the addition of kerosene to the ore as ground in water reduces the precipitating power. The graphite is then skimmed as a froth from the top of the thickeners.
Flotation has also been successfully used to treat graphitic ores. The graphite may be floated or depressed. Details are contained in Ore Dressing Notes No. 9, American Cyanamid Co., January 1939; also TP 481 of the U. S. Bureau of Mines, by Leaver and Woolf.
Gold and Silver Test Work
From the above information it will be considered that the principles of cyanidation are simple. However, every ore is different and to learn the characteristics of the particular ore before a mill is built it is necessary that thorough test work be conducted. The cost of this test work will be the cheapest item of the expense of the mill. Samples must be representative of the ore to be treated and from a tonnage, metallurgical and chemical viewpoint.
Images https://www.birkey.com/technical-illustration/how-gold-is-made-newmont-gold/
Source: This article is a reproduction of an excerpt of “In the Public Domain” documents held in 911Metallurgy Corp’s private library.
