One of the more common classes of ores containing gold is when gold is associated with pyrite, pyrrhotite, and arsenopyrite. This is the area of gold recovery that has probably received the most research and plant optimization support especially in light of the long history of South African industrial practice. From a reagent viewpoint, charged water soluble collectors such as xanthates, dithiophosphates, some thiophosphate analogs including phosphinates, and some recent adaptations of nitrogen based collectors starting from either urea and/or amines, are the most successful reagents used, e. g. Klimpel (1994). Pyrite as it occurs in sulfide mineral systems requires a collector to float. There is a great deal of fundamental research currently going on in the flotation of pyrite with various chemical structures.
Experience has shown that in the acid pH range of 4 to 5, almost all standard thio collectors are effective as pyrite collectors. Xanthates are not stable at these acid pH’s, e. g. Harris (1988), so that they are used in pyrite flotations only at alkaline pH’s. Collectors such as mercaptobenzothiazole, dithiophosphates, monothiophosphates, thionocarbamates, and dialkly sulfides all have the capability of functioning effectively in neutral to acid side flotations.
Figures 8 and 9 show a recent data set generated by this author on an ore having gold associated with pyrite from South America. Th pH in this data set is controlled by sulfuric acid addition on the acid side and lime on the alkaline side. All experiments were carried out in a two liter batch flotation cell using a blended frother of polypropylene glycol methyl ether components ranging in molecular weight from 200 to 400. The frother dosage was held constant at 35 g/Mton and the grind size was 78% less than 75 microns. The various collector

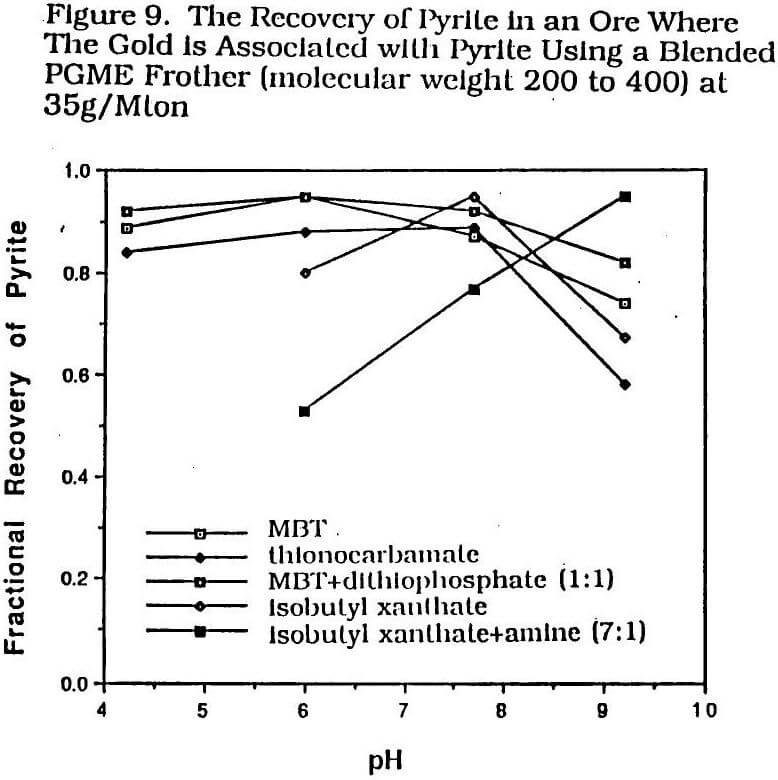
dosages were adjusted to give approximately the same maximum gold recovery capable with each collector. The mercaptobenzothiazole (MBT) at 80 g/Mton, the ethyl isopropyl thionocarbamate at 40g/Mton, and the mixture of MBT and di-isobutyl dithiophosphate at 80 g/Mton were each added to the grind. The isobutyl xanthate at 120 g/Mton and the mixture of isobutyl xanthate and the thio ether amine, C6H13SCH2CH2NH2, at 80 g/Mton were added to the cell.
Figures 8 and 9 show a number of trends that this author has found to be valid over the ten studies of various ores having gold associated with pyrite. The first trend is that it is possible to develop a large number of different reagent schemes (mostly collector blends) for such ores regardless of the desired pH range of operation. On the alkaline side, a xanthate is probably the most effective major collector component to use. On the acid side, a mercaptobenzothiazole is probably the most effective major collector component to use.

The second trend has already been implied and is the observation that the maximum gold recovery in associated pyrite ores is always achieved by a blend of two or more collectors. The dithiophosphates, especially di-isobutyl dithiophosphate, are particularly good blending collectors for gold associated with iron sulfides. With such ores, almost any collector blend is preferred over a single collector. More recently, some new and specialized collectors developed by a number of companies are being used for such blending. For example, on the acid side, monothiophosphate is showing promise and on the alkaline side, nitrogen containing collectors in blends have given good results, e. g. Klimpel (1994).
The third trend is that in order to achieve high gold recovery in systems where gold is associated with pyrite, it is generally necessary to maximize the recovery of pyrite. For example, in Figure 9 even a recovery of 90 plus % of the pyrite only achieves an 80 plus % of gold recovery. In this author’s plant experiences, the dropping off of the pyrite recovery of even a few percent can significantly impact the gold recovery. This situation almost always implies high reagent dosages, sometimes the use of an activator(s), generally very specific control of pH, and lots of flotation capacity to handle the large tonnages of concentrate.
When the goal of a pyritic gold flotation is to produce sulfuric acid by roasting the concentrate, the need to maintain sufficient pyrite grade for self-roasting can result in significant gold losses. Achieving high gold recovery and high sulfur pyrite concentrate grade are usually incompatible goals and, like so many other flotation recovery-grade trade-offs, achieving an acceptable economic balance can be difficult. There really isn’t a simple reagent solution to this balancing problem other than to treat each ore as a unique case and to avoid reagent overdosing whatever the collector scheme chosen.
The recovery of gold associated with arsenopyrite and pyrrhotite has a similar response pattern from both collector and pH viewpoints as illustrated in Figures 8 and 9. It is sometimes desired to recover arsenopyrite away from pyrite. This is also an active area of flotation research and clearly the regulation of flotation pulp electrochemistry has a major bearing on the efficiency possible with this specific separation. The idea here is to find an electrochemical window where collector adsorption occurs on one mineral species, e.g. arsenopyrite, and not on another mineral species, e. g. pyrite. Very preliminary work with chelation oriented collectors also shows some promise in this type of separation. In this author’s experience, arsenopyrite/pyrite systems have a tendency to be unique to a given ore body so that what works with one ore does not always work well with other ores.
Copper sulfate is often used as an activator in the flotation of gold associated with iron sulfides. Experimentally, when copper sulfate is used in the flotation of gold bearing pyritic ores, the recovery is higher and the rate of flotation is faster. The use of copper sulfate is particularly effective in improving relatively coarse particle flotations involving pyrite. The adsorption of copper sulfate onto pyrite is higher at neutral to acidic pH’s than in the alkaline region. With regard to dosage, too much copper sulfate causes froth instability.
