Table of Contents
The following method of testing the precipitation of gold in pregnant cyanide solutions has been adopted by the Ore Dressing and Metallurgical Laboratories, Bureau of Mines, Ottawa. The apparatus consists of three Erlenmeyer flasks of 3000-cc capacity (see Fig. 5). Flask A is for evacuation of air from the pregnant solution, flask B is for precipitation, and flask C is for the barren solution. These flasks are connected in series by tubing and are airtight. The “barren- solution” flask C is connected to the suction.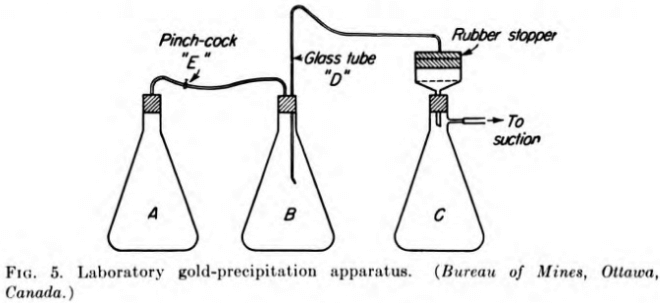
Filtering apparatus for gold precipitate
The Buchner filter is connected to a suction, and three sheets of filter paper are placed on the perforated bottom of the filtering funnel. The filter paper is covered with a layer of asbestos. The latter is packed down and then covered with a layer of about 1/16 in. of diatomaceous silica. A small piece of glass is placed on top of the layers below the opening in the rubber stopper; this prevents the solution entering the filtering apparatus from washing out a depression in the asbestos and diatomaceous silica layers.
In the “evacuation” flask A is placed 330 cc of clarified pregnant solution. The flask is shaken by agitation apparatus in such a manner as to give the solution a centrifugal motion. This type of motion spreads the solution along the walls of the flask, thus exposing a larger surface area and also a thin layer of solution to the vacuum. When the air has been evacuated from the solution (this point is readily observed by the disappearance of froth caused by the agitation), the flask is turned over and the solution passed into the “precipitation” flask B, in which has been placed a known amount of zinc dust, and agitated for 15 min. At the end of that time, the rubber tubing between flasks A and B is clamped by means of a pinchcock E and the connection broken between the two flasks. The glass tube D connecting flasks B and C is pushed down to the bottom of the flask, and a small amount of air is admitted into the flask B by means of the pinchcock E. This lowers the vacuum in flask B, and the solution passes through the filter into flask C.
This method is not comparable to the Merrill-Crowe method used in mill practice. Hence it is necessary to run comparative tests against ores which are known to give very low barren solutions in mill practice.
Gold Settling-test Procedure
The principles of settling-test procedure and the application of formulas to determine sizes of continuous thickeners are relatively simple and are briefly described below. However, detailed manipulations under specific problems and the application of test results to successful milling practice call for experienced interpretation.
The following formula expresses the relationship between the settling rates of pulp at various dilutions, in terms of thickener area required:
A = 1.333(F-D)/R x sp. gr.
where A = square feet per ton of dry solids per 24 hr.
R = settling rate in feet per hour of a feed with F dilution
sp. gr. = specific gravity of liquid
F = weight ratio of liquid to solids for the rate R
D = weight ratio of liquids to solids in discharge
The zone requiring the greatest unit area is found by applying this formula to pulp of different densities, ranging in dilution from feed to discharge density. It is this zone winch determines the area that must be provided for the pulp being tested. It is important that settling tests to determine the size of equipment for a cyanide circuit be carried out in cyanide and lime solution, following the actual period of agitation desired and using the required amounts of cyanide and lime.
Thickening Capacity
The following formula is used to determine the volume provided in a tank in the thickening zone. Such volume depends directly upon the period of detention required for the sludge to reach the desired density:
V = 4T(G – sp.gr.)/3G(S – sp. gr.)
where V = volume in cubic feet required for thickening per ton of solids per 24 hr.
S = average specific gravity of thickened pulp during compression period
sp. gr. = average specific gravity of solution
G = average specific gravity of solids in pulp
T = period of detention in hours
Reagents
Cyanide pulps are usually flocculated to some degree owing to the maintenance of normal protective alkalinity, and this results in line settling and a fairly clear overflow. In some cases, however, additional lime or other flocculating reagent must be added, and among these, caustic starch is one of the most effective.
Starch, solutions capable of flocculating finely divided solids suspended in water can be prepared either by heating under pressure in the range of 100 to 160 °C. or by causticizing starch paste. Maximum efficiency with a non caustic solution is attained when the reagent is prepared at 140 to 145°C. Causticizing temperature depends on the strength of caustic solution used. At 25 °C an efficient reagent can be produced with a 2.5 per cent solution of commercial NaOH. Starch reagent can be prepared most economically by causticizing or heating a 5 per cent starch paste with thorough mixing and diluting. Any starch can be used to prepare a flocculating reagent, but potato starch is recommended. Solutions prepared by heat alone will retain their properties for 3 days. Causticized solutions retain their properties for 2 weeks or longer. The use of these flocculating reagents appears to have a wide range of application.
Filter Tests and Calculation
Table 4 illustrates the technique of laboratory filter tests and the deductions gained therefrom. The conditions were an ore pulp of 42 per cent moisture content, filtered on a leaf of 0.5 sq. ft. capacity at room temperature. The objective was a reasonably dry cake with maximum capacity and a filter to handle 100 tons of dry solids per day.
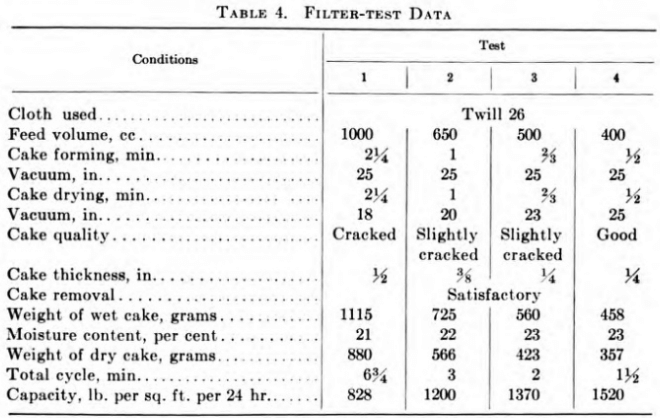
The results of tests 2 and 3 were selected, and, a 40 per cent safety factor being allowed, a filter area of 220 sq. ft. for 100 tons per day was recommended. The factor of safety to apply to any individual test is a matter of judgment based on experience and will vary considerably.
