Table of Contents
Aurous Oxide, Au2O
This gold oxide is prepared by decomposing aurous chloride, AuCl, or the corresponding bromide by potash in the cold (Berzelius) when a violet precipitate forms, which is blackish when moist, but greyish when dry. When freshly precipitated it is soluble both in alkalies and in cold water, forming an indigo blue solution, with brownish fluorescence, and on warming the solution slightly the corresponding hydrate is precipitated. It is also prepared by the action of nitrate of mercury on the trichloride, and by boiling aurate of potash with organic compounds, such as citrates or tartrates, or by boiling a solution of the trichloride with the potassium salts of these acids. When prepared according to these methods, aurous oxide always contains a certain proportion of metallic gold. The oxide may be obtained pure by reducing brom-aurate of potassium at 0° by SO2, passing in the gas only until the solution becomes colourless, after which an excess of gas would have precipitated metallic gold. Aurous hydrate is then precipitated by potash, and, after being agglomerated by boiling, it is filtered, washed with cold water, dried, and heated to 200° to expel the water of hydration. At 250° it is resolved into gold and oxygen. Hydrochloric acid decomposes aurous oxide into metallic gold and auric salts, slowly in the cold, quickly at a boiling temperature; aqua regia dissolves the oxide, but sulphuric and nitric acids are without action on it, while weak bases at once decompose it.
 An intermediate oxide, AuO, is prepared as a black powder by dissolving metallic gold in aqua regia containing an excess of hydrochloric acid, then adding an excess of carbonate of potash, and afterwards filtering and drying the precipitate. It has been little studied, but the temperature at which it decomposes has been fixed at 205° and its hydrate has been prepared.
An intermediate oxide, AuO, is prepared as a black powder by dissolving metallic gold in aqua regia containing an excess of hydrochloric acid, then adding an excess of carbonate of potash, and afterwards filtering and drying the precipitate. It has been little studied, but the temperature at which it decomposes has been fixed at 205° and its hydrate has been prepared.
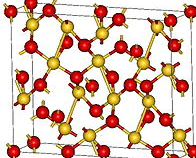
Auric Oxide, Au2O3
This is, the best known gold oxide, is a black powder when anhydrous, and is precipitated from solutions of auric chloride in the form of a hydrate by the caustic alkalies, the carbonates of the alkalies, and hydrates of the alkaline earths or zinc. The readiest method of preparation of this compound is to add caustic potash, little by little, to a hot solution of gold chloride, until the yellow precipitate of auric hydrate, Au(OH)3, first formed is dissolved to a brown liquid which contains potassium aurate, KAuO2, Then a slight excess of sulphuric acid or some Glauber’s salt is added, the precipitate filtered off, washed and purified from potash by being redissolved in concentrated nitric acid, and reprecipitated by dilution with water. On drying this precipitate in vacuo, the hydrate Au2O3 . H2O, an ochreous powder, results. If it is heated to 110°, oxygen begins to be given off. At 160°, AuO remains, and on heating for some time at 250°, metallic gold remains. Tri¬oxide of gold dissolves in concentrated sulphuric and nitric acids, from which it is partly reprecipitated on boiling or on dilution, and these solutions are supposed to contain sulphates and nitrates of gold respectively. Double nitrates of gold and the alkalies have been obtained as crystals. Hydrochloric and hydrobromic acids dissolve the trioxide forming the haloid salts, but hydroiodic acid decomposes it on boiling, giving iodine and metallic gold. Gold trioxide dissolves in boiling solutions of alkaline chlorides, giving aurates and chlor-aurates, while it also combines with metallic oxides to form aurates.
It is easily reduced by hydrogen, carbon and carbonic oxide, with the aid of very gentle heat. Boiling alcohol reduces it, yielding minute spangles of gold which were formerly used in miniature painting.
Aurates
The aurates of potash and soda have the general formula Au2O3.R2O or R’2Au2O4 assigned to them. They are readily soluble, crystallisable compounds, and are formed when alkalies are added in excess to solutions of gold chloride. The aurates of calcium, magnesium and zinc are insoluble in water, but soluble in hydrochloric acid.
Fulminating Gold
Fulminating Gold is a compound of auric oxide with ammonia, Au2O3(NH3)4, which is formed by precipitating gold chloride with ammonia or its carbonate, or by the action of ammonia on gold trioxide. When prepared by the former method its composition is variable, but the fulminate is always a high explosive decomposing with violence at 145°, or on being struck, and sometimes even spontaneously. It is decomposed without explosion by sulphuretted hydrogen, and by protochloride of tin. It is a grey pulverulent powder, insoluble in water, but soluble in potassium cyanide, auricyanide of potassium being formed.
