Experience has shown how difficult it is to obtain information regarding laboratory-tests in connection with the gold ore chlorination process for the extraction of gold from its ores, and I therefore present the following method, somewhat in detail, for the benefit of those who may desire to pursue research work in this field.
The ore chosen was a partly-decomposed porphyry, extremely siliceous and comparatively soft, and of an average value per ton of from $25 to $26 in gold and $0.68 in silver. The chemical analysis gave: Fe203, 3.35; FeS, 1.5; MnO, 0.75; ZnC03, 4; Al203, 3.20; H20, 0.7 ; Si02 and insol., 83.50 per cent.
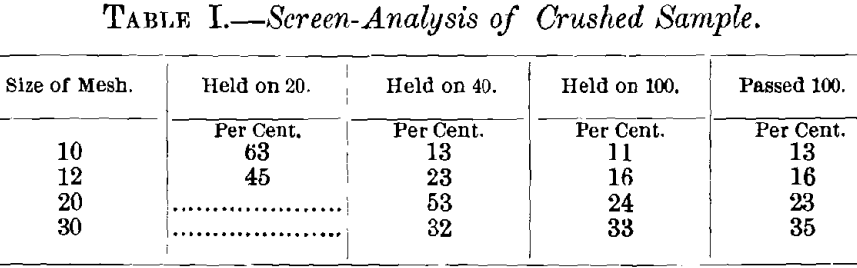
The ore, first crushed to pass a 5-mesh screen, was coned and quartered until a 45-lb. sample was obtained. Samples for assay and analysis, taken from this lot by means of riffles, were crushed to pass a 100-mesh screen. The original sample was divided into 10-lb. lots, of which one was crushed to pass a 10- mesh screen; another through a 12-; the third through a 20-, and the last through a 30-mesh screen.
Because of the production of an excessive quantity of fine material it was deemed inexpedient to crush finer than 30-mesh size—a decision the wisdom of which is proved by the data in Table I., showing the increasing ratio of slimes, in proportion as the ore is crushed finer.
In order to determine the percentage of fines in the sizes of mesh selected, a weighed sample of ore was taken from each mesh, and a sample from the 10-mesh lot was passed successively through 20-, 40- and 100-mesh screens, and the percentage remaining on each screen determined. This process was repeated with the 12-, 20- and 30-mesh lots, with the results given in Table I.
II. Gold Ore Roasting:
When the analysis was complete, some of the ore was chlorinated in a manner to be explained later, and it was found that no practically important extraction could be obtained, due to the fact that the sulphur absorbed a large part of the chlorine and that the gold was not in a suitable form to be attacked by chlorine. In order to reduce the sulphur to a working-limit (about 0.2 per cent.), it was necessary to roast.
For preliminary roasting, a small amount of ore in scorifiers was put in a muffle and the heat gradually raised, the total time of roasting being 2, 3 and 4 hr. Table II. shows that the ore was roasted within the 0.2 per cent, limit of sulphur at the end of 3 hr., and dead roasted at the end of 4 hr.
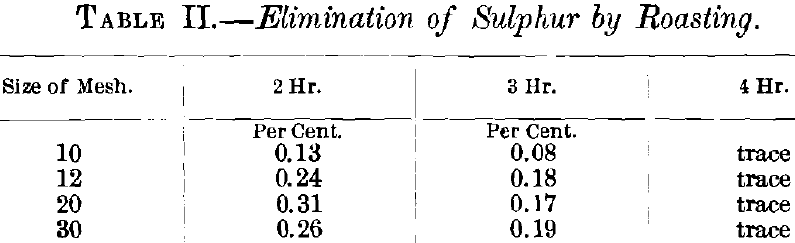
The 3-hr. roast was taken as the one on which to work, and in roasting the ore for the chlorination-tests, roasting-dishes were placed in a muffle, the door of which was kept open, the heat being gradually raised to redness; the endeavor being to maintain as great a heat as possible without sintering the ore. A number of results obtained from the 3-hr. roasts were not altogether satisfactory, and the time of roasting was lengthened to 4 hr., which practically corresponds to a dead roast.
III. Gold Ore Chlorination:
The chlorination proper was carried out in the following manner in soda-water bottles: The chloride of lime was first weighed and charged. Then the 100 g. of ore was introduced, and water in sufficient quantity to give the ore the consistency of mud. Finally, the sulphuric acid was charged and the bottle sealed by pulling the patent stopper in place, inserting a wooden wedge to tighten it, and tilling the neck with melted paraffine. It was necessary to conduct this operation with care and rapidity, and to avoid all agitation, because the chlorine soon began to bubble up through the paraffine if the latter did not solidify immediately. It is advisable to have the bottles perfectly dry before charging the chloride of lime, so as to avoid the premature generation of chlorine.
The bottles were placed in an agitator, and rotated for the required time. This agitator consists essentially of a circular disk 2 in. in thickness and 12 in. in diameter. The bottles were secured by rubber bands in six semicircular notches around the periphery. The disk lies in a plane inclined to its axis of rotation at an angle of 80°, and is connected by belt to a 1/10-h.p. motor, which furnished ample power; 30 rev. per min. was the average speed at which the tests were made.
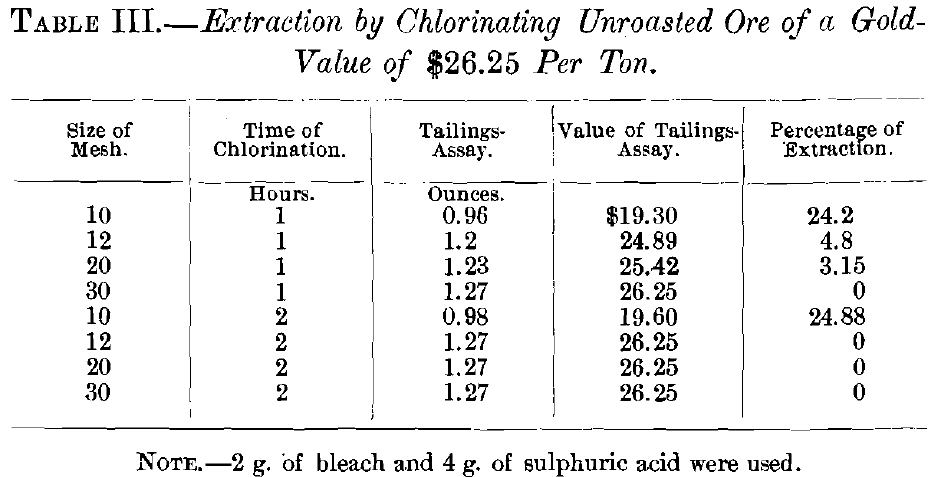
In the first series of experiments, on unroasted ore, with 2 g. of chloride of lime and 4 g. of sulphuric acid, the samples were agitated for 1 and 2 hr. by hand. In the second and all subsequent tests the agitator was used. The second series was on ore roasted for 3 hr., with 2 g. of chloride of lime and 4 g. of sulphuric acid, the agitation lasting 1, 2, 3, 4 and 5 hr., respectively. These gave results which, though a great improvement over those on unroasted ore, were not entirely satisfactory.
IV. Gold Ore Leaching:
In each case, as soon as the time of chlorination was complete, the bottles were opened and the excess chlorine allowed to escape; then the ore was leached or filtered in sand-filters constructed as follows: A common acid-bottle, upside down, with the bottom removed and a stopper with a pinch-cock inserted in the neck, formed the percolator. Pure quartz was crushed and screened to pass 10-, 20- and 30-mesh screens, respectively. A few pebbles larger than 10-mesh size were placed in the bottom, above which was a layer 1 in. thick of quartz of 10-mesh size, then 0.75 in. of 20-mesh, and lastly 0.5 in. of 30- mesh size, and above the uppermost layer was placed a cloth, to catch the ore, and thus preserve the tailings. Too much fine sand prolonged the term of filtration excessively, while too much coarse material allowed the fines of the ore to pass through the filter. It was found impossible to recover all the fines in the tailings for assay-purposes, since a portion passed through the cloth. In some cases a portion of the fines was caught in the sand, while in others the fines passed into the filtrate. It was found that to remove all traces of gold chloride from the tailings each 100-g. charge required about 700 cc. of wash-water, which is equivalent to about 14,000 lb. of water per ton of ore. In practice this amount of water would be considered excessive, and would increase the number of settling-tanks and precipitating-vats to such an extent as to increase seriously the working-cost. The small sand percolator acts like the paper filter used in chemical work, the water being drawn through the cloth along the glass, without coming in contact with the ore, while in the barrel the water must pass through the whole body of ore before reaching the filter. In the mill a further economy of wash-water is often effected by using the last washing of one barrel for the first washing of the next barrel. The washing is considered complete when, on passing hydrogen sulphide through the wash-water, no gold sulphide is precipitated.
V. Treatment of Tailings:
As soon as the leaching is complete, the cloth containing the tailings is removed and the tailings dried, bucked to pass an 80-mesh sieve and assayed. The difference from the original assay is the amount extracted by the chlorine, and may be calculated in terms of per cent, as follows:
Gold before treatment……………………………1.27 oz. per ton.
Gold after treatment………………………………0.96 oz. per ton.
0.31
1.27 : 100 : : 0.31 : x = 24.2 per cent., etc.
The actual extraction was slightly higher than that indicated by the results obtained in the experiments on account of the inaccuracy of the tailings-assay, which arose from the passage of some of the fines (obviously thoroughly leached) through the cloth, thus giving a higher result for the tailings-assay.
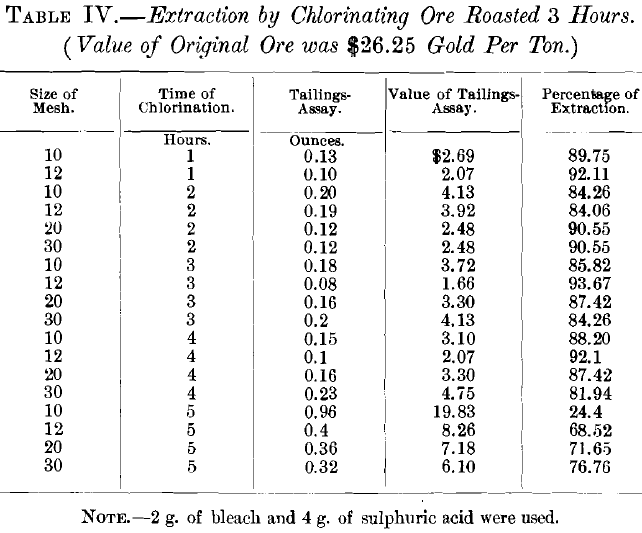
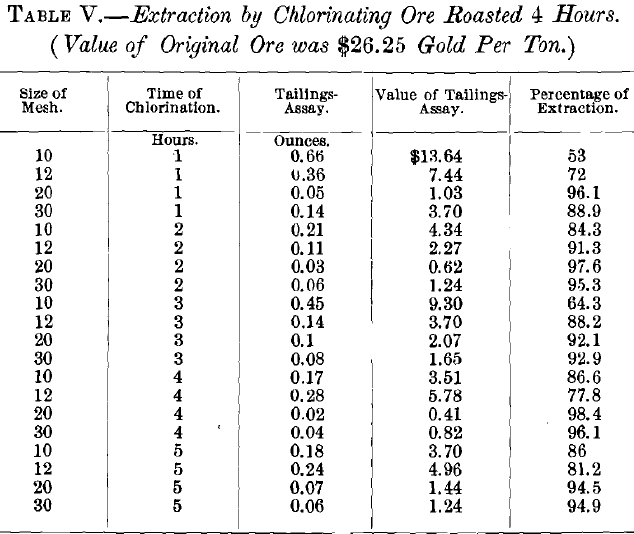
VI. Discussion of Tests:
The results of the experiments on ore roasted for 3 hr. were unsatisfactory, since the best extraction was only 93.67 per cent. Assuming that the poor extraction was due to the 0.2 per cent, of sulphur left in the ore, experiments were then made on ore that had been roasted 4 hr., which entirely eliminated the sulphur. At the same time the quantity of bleaching powder was increased from 2 to 3 g., and the quantity of sulphuric acid from 4 to 6 g. Under these conditions the results were entirely satisfactory, the best extraction being 98.4 per cent. The percentage of extraction varied with both the size of the ore and the time of chlorination. The former showed an average extraction as follows: 10-mesh size, 72.84 per cent.; 12- mesh, 82.1 per cent.; 20-mesh, 95.7 per cent.; and 30-mesh, 93.6 per cent. With regard to the time of the experiment, the average extraction was: 1 hr., 77.5 per cent.; 2 hr., 92.125 per cent.; 3 hr., 84.37 per cent.; 4 hr., 89.7 per cent.; and 5 hr., 89.15 per cent. The best single test was with 20-mesh size and 4 hr. chlorination, using 3 g. of bleach and 6 g. of sulphuric acid. Accordingly, this size and time were adopted, leaving only the quantity of bleach and of acid to be determined.
VII. Tests for Quantity of Bleach and of Sulphuric Acid:
Using ore of 20-mesh size and chlorinating for 4 hr., the results obtained by varying the quantity of sulphuric acid and of bleach are given in Table VI.
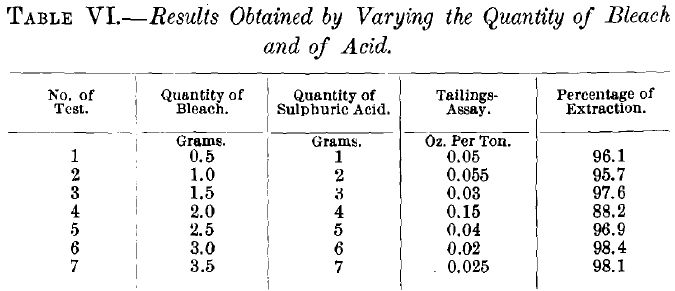
The results given in Table VI. show that the first, third and sixth tests are the only ones worthy of consideration. Test No. 1 required 10 lb. of bleach and 20 lb. of sulphuric acid per ton of ore. The cost of 10 lb. of bleach at 1 c. is $0.10, and of 20 lb. of sulphuric acid at 0.6 c. is $0.12, giving a total cost of $0.22. Test No. 3 required 30 lb. of bleach and 60 lb. of acid, which cost $0.66. No. 6 required 60 lb., and 120 lb., costing $1.32. The value of the gold extracted in Test No. 1 was: $26.25 x 0.961 = $25.22; No. 3, $26.25 X 0.976 = $25.62, and No. 6, $26.25 x 0.984 = $25.83.
Balancing the increased extraction of gold against the increased cost of chemicals, Test No. 1 was the most economical. In the barrel-test based on Nos. 1 and 3 the latter proved to be the better. The most satisfactory results of these tests show that the ore should be 20-mesh size, the time of the roast 4 hr., the quantity of bleach 30 lb., and the quantity of acid 60 lb., per ton of ore treated.
VIII. Treatment of the Gold Chloride Solution:
The gold chloride solution was treated with hydrogen sulphide, which precipitated the gold as gold sulphide, the solution being constantly stirred during the operation in order to facilitate settling. After complete precipitation the solution was allowed to settle for a few hours, and the clear supernatant liquid was decanted through a filter; finally, the gold sulphide was washed on this filter, dried, and roasted until all traces of sulphur had disappeared.
The gold was refined in scorifiers with test-lead, borax, and silica, the lead button thus obtained cupelled, and the gold weighed. From this weight the percentage of extraction may be determined as a check on the results obtained by the tailings- assay. This method, however, seems of little practical value because of the intricacy of the manipulation required, and the liability of loss of gold in the roasting, and in the burning of the filter-paper on which it is caught.
IX. Barrel-Test:
In order to approximate more nearly to conditions of actual practice, a chlorination-test was made in a small barrel of 100- lb. capacity, basing the experiment on the data obtained from the laboratory experiments.
An iron barrel 2 ft. long and 1.25 ft. in diameter, inside measurements, lined throughout with lead, and provided with the conventional man-hole and inlet- and outlet-valves, was used. An internal wooden frame-work was provided to support the asbestos filter.
A charge of ore, weighing 72 lb., was crushed to 20-mesh size, and roasted for 4 hr. on the floors of a series of muffles. The barrel, having the filter in place, was filled one-third full with water, to which 2.1 lb. (3 per cent, of the weight of the ore) of sulphuric acid was added. The ore was then introduced, followed by 1.05 lb. of bleach (1.5 per cent, of the weight of the ore). The man-hole and valves were quickly and securely closed, and the barrel was rotated for 4 hr. At the end of this time the rotation was stopped, with the filter underneath; the valves were opened and the leaching began. Atmospheric pressure being insufficient to cause rapid filtering, on account of the fines which formed a cement-like cake over the top, compressed air was used to facilitate the leaching. When the leaching was complete, the barrel was closed, rotated a few times, and then opened and emptied. The tailings-sample was taken, when the leaching was finished, by means of a short iron pipe, which was plunged into the ore at different places. Three check-assays gave an average of 0.083 oz. of gold per ton (or $1.74), which shows an extraction of 93.46 per cent.
During the leaching, 50 gal. of wash-water was used, which is equivalent to 1,338 gal. per ton of ore. This large excess was due to the care taken to remove the last traces of gold chloride from the ore.
These experiments, both laboratory and barrel, demonstrate the suitability of the ore for treatment by chlorination. The original ore assayed $26.25 in gold and $0.68 in silver per ton. Assuming the extraction obtained in the barrel-test to be 93.46 per cent., there was a loss of value of $1.74 in gold, and all of the silver, making a total loss equal to $2.42 per ton.
The smelter-charge for an ore of this character is about $10 per ton, and neglecting the cost of eliminating the sulphur (which in general is in favor of the chlorination-process), there is a gain in chlorination above smelter-charge of $10, less $2.36, equal to $7.64 per ton.
Ores of the character mentioned above can be chlorinated at a cost of from $2 to $5 per ton, which gives a substantial gain over the cost of treatment in a custom-smelter.
Chlorination of Gold-Ores; Laboratory-Tests, BY A. L. SWEETSER
