Table of Contents
Sodium cyanide and Nokes reagent are commonly used depressants to reject iron, copper, and lead sulfide minerals for the flotation recovery and upgrading of molybdenite concentrate. With the ever increasing cost of reagents and more stringent regulations, it is necessary that these depressants are used as efficiently and effectively as possible. Therefore, a study was undertaken to investigate the effects of concentration and conditioning time of two depressants—sodium cyanide and Nokes reagent—at different levels of pH on the rejection of sulfide impurities from the molybdenite concentrate. This paper discusses the experimental methods used and the results obtained to date on this ongoing investigation.
A representative sample of the first-stage cleaner flotation feed in the mill was obtained for this study. The sample assayed 6.2 percent MoS2, 13.5 percent FeS2, 0.108 percent Cu, and 0.026 percent Pb. The bench-scale flotation tests were performed in a 4.5-liter Denver D12 flotation machine. Concentrates were collected at cumulative times of 3, 5, and 7 minutes of flotation, and flotation products were assayed for MoS2, Cu, Pb, and FeS2.
Three series of tests were performed to investigate the following:
- effect of conditioning time of depressants,
- effect of pH, and
- effect of depressant addition rates on the rejection of copper, pyrite, and lead impurities present in the molybdenite concentrate.
The results presented were analyzed at a constant concentration ratio of 5. This value of the concentration ratio was selected because the recovery of molybdenite obtained in the laboratory was equivalent to the recovery obtained in the first-cleaner flotation circuit.
Effect of Conditioning Time
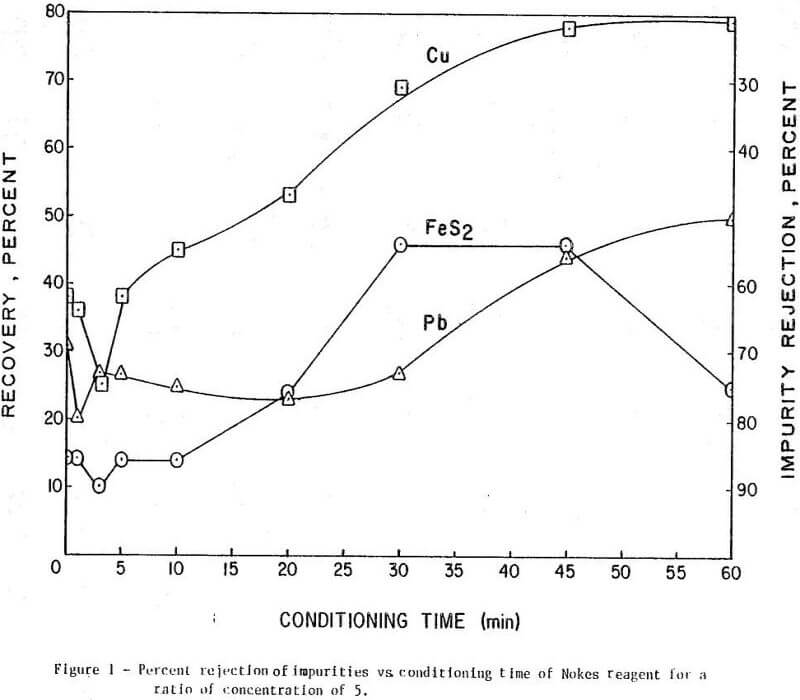
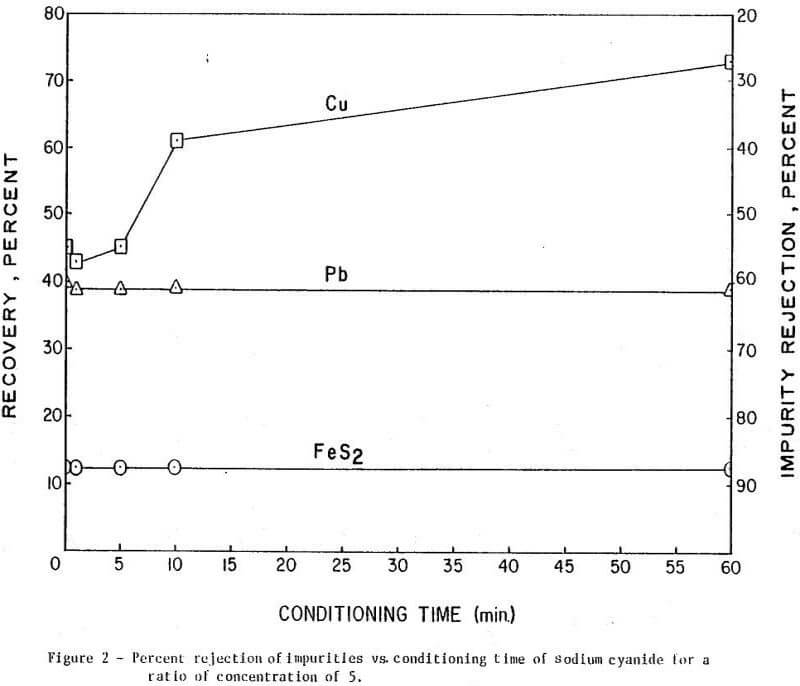
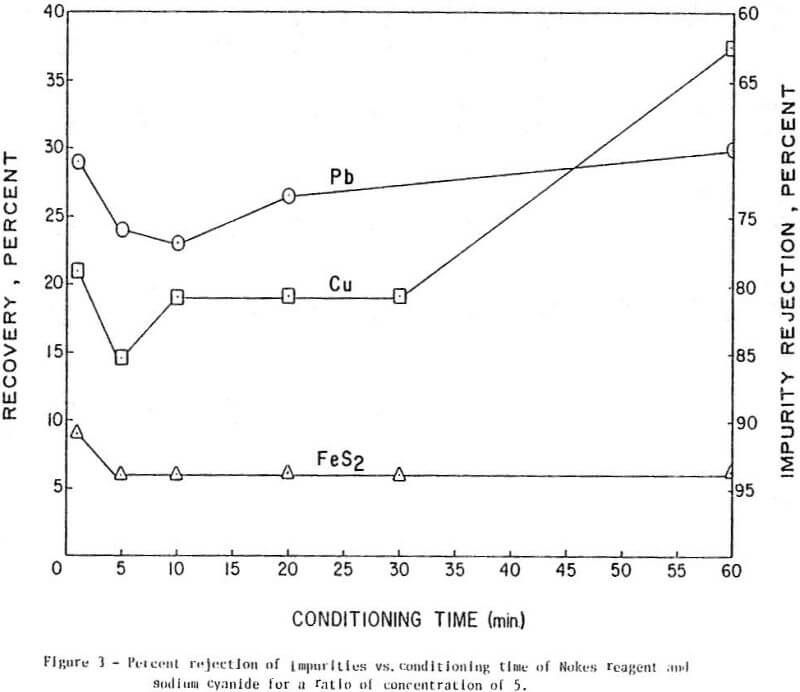
The results of conditioning the pulp with 0.25 lb/ton of Nokes reagent alone at different conditioning times are presented in Figure 1. In Figure 2 is presented the results of conditioning the pulp with 0.30 lb/ton sodium cyanide only at different conditioning times. The results of conditioning the pulp with the combination of Nokes reagent (0.25 lb/ton) and sodium cyanide (0.30 lb/ton) at different conditioning times are shown in Figure 3.
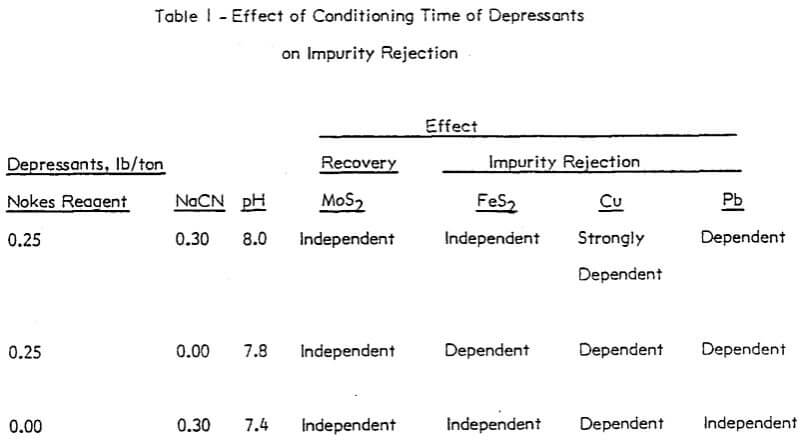
The trend of the effect of conditioning time of depressants is summarized in Table 1. The conditioning time of any reagent investigated or reagent combinations, did not affect molybdenite recovery. The rejection of impurities was dependent on conditioning time for Nokes reagent. The rejection of copper was indicated to be dependent on conditioning time of sodium cyanide. These preliminary results indicate that optimization of conditioning time for Nokes reagent or sodium cyanide is important for maximizing rejection of impurities. In this series of tests, 5-minute conditioning was found optimal for the laboratory experiments.
Effect of pH
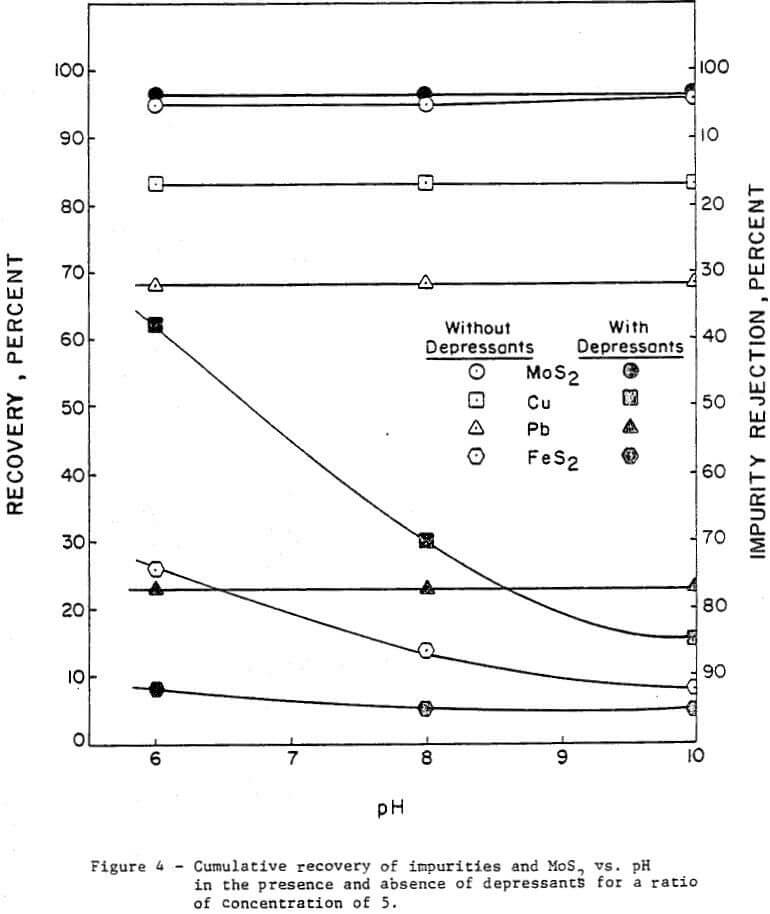
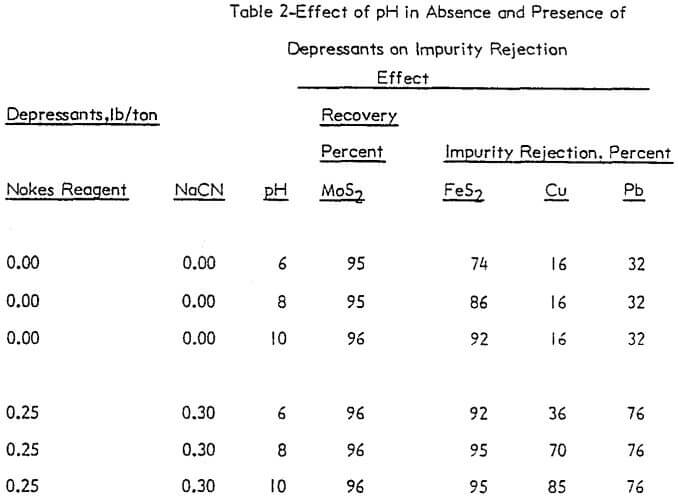
The effect of pH was investigated without depressants and with depressants (0.25 lb/ton Nokes reagent and 0.30 lb/ton sodium cyanide). The pH was adjusted with potassium hydroxide or hydrochloric acid as required. The results are presented in Figure 4 and summarized in Table 2.
In the absence of depressants, the rejection of pyrite was dependent on pH; and the rejection of lead and copper minerals was independent of pH. However, in the presence of Nokes reagent and sodium cyanide, the rejection of pyrite and lead was independent of pH. The rejection of copper was strongly dependent on pH.
Effect Nokes Reagent
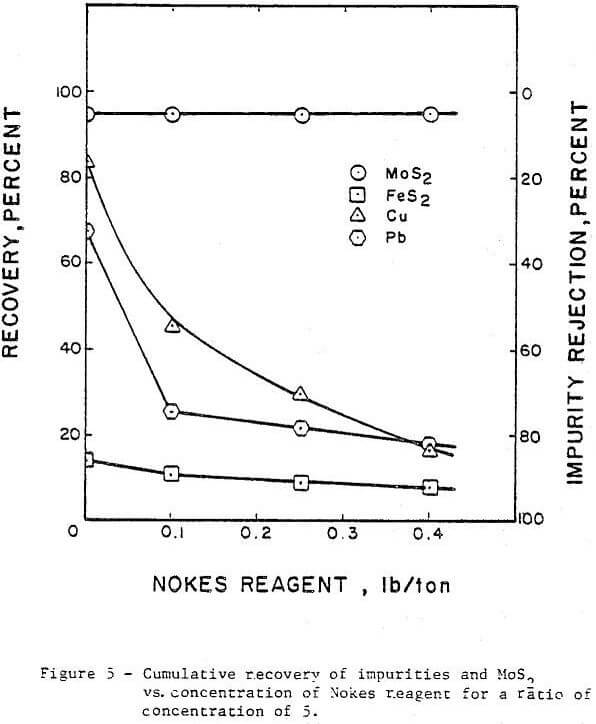
The concentration or addition rate of Nokes reagent was investigated in the range from 0.0 to 0.4 lb/ton with the pH adjusted to 8. The results are presented in Figure 5. The rejection of copper increased from 14 percent with no Nokes reagent added to 83 percent with 0.4 lb/ton Nokes reagent added. The increase in pyrite rejection was minimal when the Nokes reagent addition rate was less than 0.1 lb/ ton. Higher addition rates than 0.1 lb/ton also resulted in little improvement in pyrite rejection. The rejection of lead improved from 32 percent without Nokes reagent to 82 percent when 0.4 lb/ton of Nokes reagent was used. In all cases, no detrimental effect was noted on the molybdenite recovery. However, bubbly froth which is difficult to control was observed when the Nokes reagent concentration was increased beyond 0.3 lb/ton. This frothing problem was not observed when a combination of Nokes reagent and sodium cyanide was used in the circuit.
Effect of Sodium Cyanide Concentration
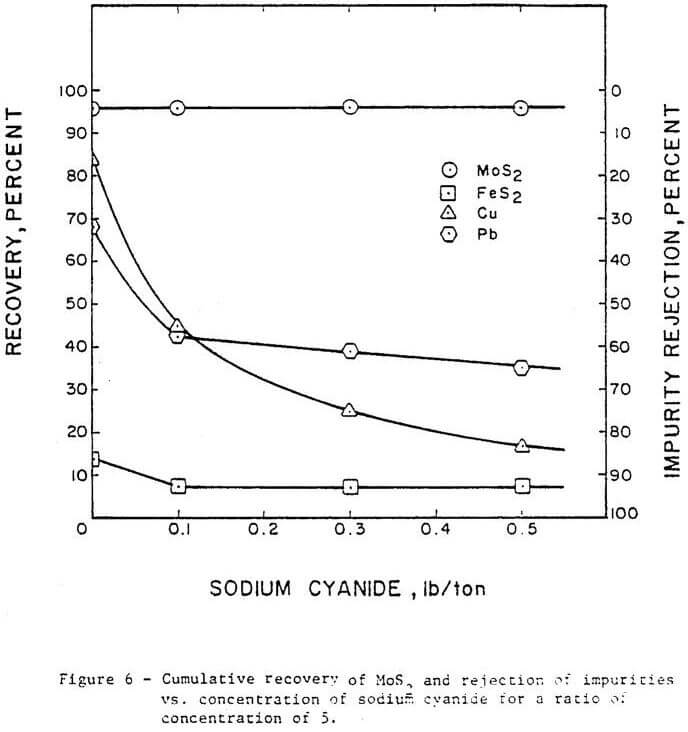
Figure 6 shows the effect of varying sodium cyanide concentration on the rejection of impurities. Copper rejection increased from 14 percent without sodium cyanide addition to 83 percent using 0.5 lb/ton sodium cyanide addition. Pyrite rejection improved from 85 percent without sodium cyanide to 93.5 percent with 0.1 lb/ton sodium cyanide; above 0.1 lb/ton no significant effect on pyrite is indicated. Lead rejection was increased from 35 percent without sodium cyanide to 65 percent with 0.5 lb/ton sodium cyanide. Based on these results, sodium cyanide was not as effective as Nokes reagent for depression of lead. At the range of sodium cyanide concentration investigated, no detrimental effect was indicated on molybdenite recovery.
Preliminary Mill Testing
Based on the findings of the bench-scale laboratory testwork, a mill testing program has been initiated. The objective of the program is to confirm the trends observed in the laboratory testwork and to improve the circuit performance by using the depressants as effectively and efficiently as possible.
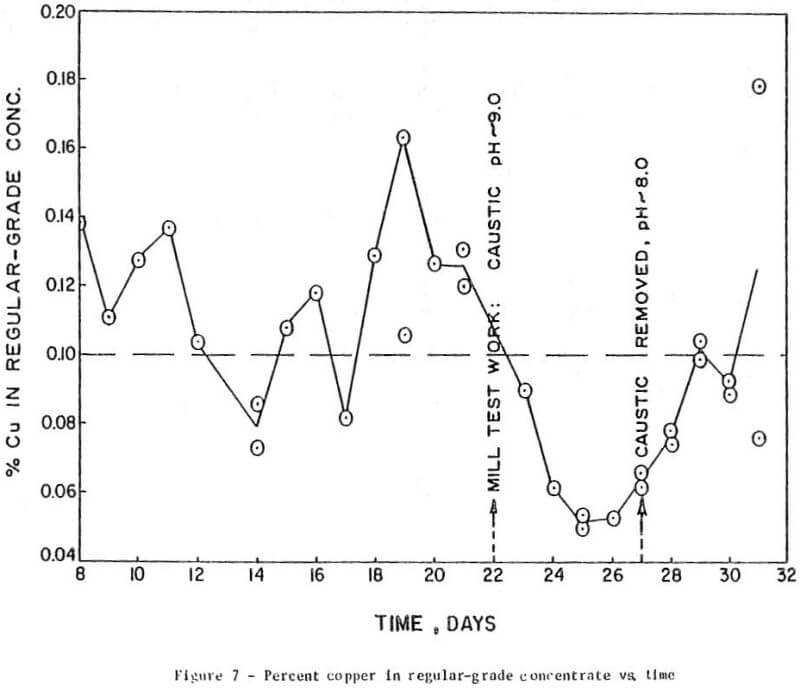
The results of preliminary mill testing, shown in Figure 7, confirmed the finding that, in the presence of depressants, the rejection of copper minerals is dependent on the pH in the flotation circuit.
Conclusions
- The recovery of molybdenite is independent of the variables investigated.
- The rejection of sulfide impurities from the molybdenite concentrate is dependent on the conditioning time of Nokes reagent.
- In the absence of depressants, the rejection of sulfide impurities, with the exception of pyrite, is independent of the pH in the flotation circuit.
- In the presence of depressants, the rejection of copper minerals is dependent on the pH in the flotation circuit. Preliminary mill testing has confirmed the trend. A pH in the range of 9 to 10 is best for maximizing the rejection of copper and other sulfide minerals.
- The use of high dosage of Nokes reagent causes frothing problems. The combination of Nokes reagent and sodium cyanide results in maximizing rejection of sulfide minerals while maintaining good frothing conditions in the circuit.
Note: Conditioning at 30 percent solids and 1200 rpm in Denver D-1 machine
Note: Conditioning at 30 percent solids and 1200 rpm for 5 minutes in Denver D12 machine