Table of Contents
This is the part of the subject of which we have heard the most; indeed, until recently the literature of the flotation process was closely identified with the records of patent litigation. That is why the scientific principles are as yet so little understood and the technology of the process has made such scanty progress. The aim of a patent specification is to disclose just enough to prove originality. In many cases this has been done to the apparent satisfaction of the Examiner of Patents without conveying all the facts essential to a clear understanding of the operations involved. The description given in a modern patent is cryptic; it is couched in a quasi-legal jargon that assists obfuscation. I refer to processes only, for the disputes over flotation patents have arisen over methods, not machines. The apparatus required had already been used in other branches of wet metallurgy, so that we have been spared one source of trouble, at least.
The litigation, which is now a serious obstacle to the free development of the process, has arisen largely from confusion of ideas as to the underlying causes of flotation. The patentees did not understand the phenomena with which they played. Those to whom they sold their patents knew even less. The interpretations of attorneys and judges have elucidated the law but confused the physics. No clear adjudication of rights is possible so long as claims and counter-claims are based on an ignorance of the rationale of the process.
As the flotation process of today is essentially that of making a mineral-buoying froth in modified water, it is not necessary for me to make further reference to the patents granted for the use of purely surface-tension effects. It would seem permissible also to omit further consideration of the bulk-oil methods, but, as a matter of fact, none of these operated without the aid of air, although the patentees were quite unaware of it, and it was from these bulk-oil methods that the frothing process was developed fortuitously.
The first patent for the use of oily substances and coal-tar products in the concentration of ores was that granted to William Haynes, an Englishman, in 1860; but this is now only of academic interest. Next comes the patent of Carrie J. Everson, dated August 24, 1886, the application having been filed on August 29, 1885. The Everson patent refers to the selective action of oil for “metallic substances” and the increase caused in that selectiveness by the addition of acid. The pulp is stirred so as to bring “the mineral” in contact with the oil and acid, producing a “stiff mass.” The use of “about a barrel of oil to the ton of ore” is mentioned, indicating a ratio of about 17%. Other statements indicate that she used as little as 5% of oil per ton of ore. The separation of the oiled mineral from the unoiled gangue is described thus: “In practice, the concentrate, after thorough agitation of the mass and detachment of the sand, will in this case be preferably removed by means of a constant overflow of water from a washing-out vessel, by which overflow the concentrate will be floated off.” These last words constitute the only direct reference to the floating of the concentrate.
A great deal more has been read into this patent than could ever have been in the mind of the patentee. It is difficult to read her description without cocking one eye at the present practice of flotation, whereby some of Mrs. Everson’s phrasing is given a significance to which it had no possible claim 30 years ago. The proportion of oil used, even the maximum, would not suffice for the operation she had in mind, namely, the floating of the heavy sulphides by direct aid of the buoyancy of oil. Her maximum proportion of oil represents a mere fraction of the quantity required for this operation. She disclosed no notion of the assistance to be obtained from air, in the form of bubbles, although, of course, this was her principal flotative agent. The process described by her is quite impracticable on a large scale, and it never was operated save in a crude experimental way. Nevertheless the exigencies of patent litigation have caused the opponents of Minerals Separation to idealize both Mrs. Everson and her metallurgical adventure, as they have also created a romantic story of the supposed epoch-making discovery. She is represented as a school¬teacher. a Miss Everson, who, as the sister of an assayer, washed some greasy ore-sacks and saw the sulphides floating on the contaminated water. Even the idea of agitation was suggested by the activity of her hands in the wash-tub. Therefore “it only required the customary acuteness of observation of the ’Western lady school-teacher to grasp the essential facts of sulphide flotation.” This is pretty, but not scientific. The “essential facts” are a bit too slippery to be grasped firmly even today. In thinking acid necessary, she was wrong. It is known now not to be an essential. Even the use of oil as a direct means of buoyancy has receded into the background; if she had understood the rationale of her own operations she would have known that it was not so much the selective adhesion of the oil to the mineral particles that gave her the requisite buoyancy as the greater selectiveness of the air bubbles made by agitation in water modified by the oil. Carrie Jane Everson had no idea of the frothing process. Her methods may have involved bubble-levitation, but she did not know it, and her description would not suggest it to anyone not versed in much later knowledge. The effort to feature this lady as the inventor of the frothing process cannot commend itself to an unprejudiced student of the subject.
It is interesting to add that the “Miss Everson” of the story was really a Mrs. Everson; the wife of a Chicago doctor; she was not a school-teacher; her brother was not an assayer; and there is no reason for regarding the story of the ore-sacks as anything more than the fiction of an irresponsible scribe. Mrs. Everson died at San Anselmo, California, on November 3, 1914.
Next comes the British patent of January 8, 1894, granted to George Robson, an Englishman, who did his experimental work at the same place and on the same ores as the Elmore brothers, at the Glasdir mine, near Dolgelly, in Wales. He disclaimed “the use of acids or salts and also the method of washing away the gangue with water,” effecting “the separation of the metallic matter by the mixture of oils alone.” He does not specify the quantity of oil, but I am informed that it was in the ratio of 3:1, three tons of oil to one of ore. This was true bulk-oil flotation and it proved an abject failure.
Then came Francis Edward Elmore, on April 10, 1899, duplicating his British patent of October 18, 1898. His method has been described already. It only remains to say that in so far as this method proved more practicable than that of Robson, the result was due to the fact that the Elmore brothers were capable engineers and therefore designed a more suitable plant. The patent ignored the use of air; the intention was not to emulsify the oil and not to aerate the pulp, but this theoretical condition was never fulfilled, as is clear from the fact that the flotative action was 150% more than that calculable from the difference of specific gravity between the oil and the water. On January 3, 1903, A. Stanley Elmore took out a British patent for an apparatus for excluding the air during the operation. He effected his purpose by sealing all the open vessels with a ring or surface of oil; from which it is evident that at that time he and his brother endeavored to base their method wholly on bulk-oil flotation.
In January 1902, Charles V. Potter, an Australian, obtained a British patent for the flotation of sulphides in a hot acid solution. He used a stirrer, and he claimed that the solution would “react on the soluble sulphides present to form bubbles of sulphuretted hydrogen on the ore particles and thereby raise them to the surface.”
In November of the same year, 1902, Guillaume D. Delprat, the manager of the Broken Hill Proprietary mine, applied for a similar patent, except that he used salt-cake instead of sulphuric acid. Litigation ensued, followed by a compromise, eliminating Potter. In later patents both Potter and Delprat introduced the use of oil, finding it beneficial.
In his first American patent, No. 735,071, filed on January 2, 1903, Delprat states that the process “depends upon the ore particles being attacked by the acid to form a gas. Each ore particle so attacked will have a bubble or bubbles of gas adhering to it, by means of which it will be floated and can be skimmed or floated off the solution.” (“Ore particles” means blende and galena at Broken Hill.) Here is a pretty good recognition of bubble-levitation, only he supposed the sulphides, not the gangue, to be attacked by the acid. In another place he says specifically: “The sulphides in the ore are rapidly attacked by the acid and gas-bubbles formed on them, that quickly carry them to the surface.” In this patent he claimed the use of nitric acid and a suitable nitrate, such as sodium nitrate, the latter being intended “to increase the specific gravity of the bath.” “What reaction was to follow between the sulphides and the dilute nitric acid is not clear. It has been recorded that in the early days of the Potter-Delprat methods it was supposed that the acid liberated hydrogen sulphide from the sulphides, when sulphuric acid was used, without attacking the gangue. Those who first scouted this idea suggested that carbon dioxide was generated by decomposition of a carbonate coating on the sulphides, due to weathering of the ore, arguing therefrom that it was necessary for the gas to be produced at the surface of the sulphide particles themselves. All of these explanations are now on the scrap-heap of discarded theories.
The Potter Flotation Apparatus

These patents of Potter and Delprat have been labelled variously under ‘acid-flotation’ and ‘surface tension’ methods. Delprat’s apparatus does indeed suggest a process of the Bradford or Wood type, but, of course, both he and Potter depended for their results on the liberation of carbon-dioxide gas from the gangue, which, at Broken Hill, contains a large proportion of carbonates, notably calcite, siderite, and rhodocrosite. From any of these a hot sulphuric-acid solution would release the gas that promptly attached itself to the metallic surfaces of the galena and blende.
Meanwhile Alcide Froment, in Italy, had got hold of the bubble idea, which is the real basis of the flotation process as it is understood today. He invented his method in 1901 and filed his claim for a British patent on June 9, 1902. This patent was duplicated in Italy, but not in the United States. The fact last mentioned is important. Froment claimed that his process was “a modification of what is known as the oil process of ore concentration,” meaning that of Elmore, for the bulk-oil method had been tried at the Traversella mine, in Italy, where Froment was engaged as an engineer. His plan was to generate bubbles of gas by the reaction between sulphuric acid and the carbonates in the gangue, adding limestone when the ore did not contain enough carbonate. He argues that “if a gas of any kind is liberated in the mass, the bubbles of the gas become coated with an envelope of sulphides and thus rise readily to the surface of the liquid where they form a kind of metallic magma.” It will be noted that he says “gas of any kind.” As the children say, in a familiar game, he was “very warm” just then, for he had only to invoke the aid of air to have described the essential principle of the later phase of flotation. He also states that the sulphide particles when “moistened by a fatty substance” have a tendency “to unite as spherules and to float upon the surface of the water.” His brief description closes with the statement that “the rapidity of the formation of the spherules and their ascension is in direct ratio to the quantity of gas produced in a given time.” As to the oil, the only mention of quantity is made in describing a test-tube experiment in which he uses “a thin layer of oil.” This phrase has been variously interpreted, according to the exigencies of litigation, but it refers obviously to a minute proportion.
In the directions given by him to the Minerals Separation people when they bought his patent rights on November 17, 1903, he specified 1% of mineral engine-oil for ore containing up to 5% of metal, 1½ of oil for ore containing 10%, and so on, up to ores containing 50% of metallic lead, which would require 3½% of oil. As oil flotation was understood at that time, this marked a great reduction in the proportion of oil. However, the more interesting point is Froment’s failure to perceive the possibility of using air as the gas for making his bubbles. He depended upon chemical action to furnish him with the necessary gas. Nevertheless Froment deserves a high place in the roll of flotation pioneers, for he made an important step forward. He furnished the link between bulk-oil and air-froth flotation.
The next chapter in the story marks a retrogression. Under date of November 28, 1902, Arthur E. Cattermole obtained British patents No. 26,295 and 26,296, both of which were acquired by John Ballot and associates in December 1902, preparatory to the formation of the company—Minerals Separation, Ltd.—organized to exploit them. In August 1903 Cattermole revised and amplified his previous claims in British patent No. 18,589, which was duplicated in the United States under date of September 28, 1903, as No. 777,273. This last is the principal patent covering the so-called granulation process.
Cattermole prefaces his description by reference to the selectiveness of oil, when emulsified, for sulphide particles, such selective action being intensified by the acidulation of the water. He then proceeds to say that if the mixture be thoroughly agitated, there is a tendency for the metalliferous particles, now coated with oil, to adhere together, forming granules that sink and are readily separated from the lighter gangue by an up-current of water. In his description of the operation he says that “the granules, with a certain amount of heavy sands, sink to the bottom and are discharged
[see Fig. 11] through a pipe G¹ into the vessel A5, while the lighter sands are carried away by the upward current and discharged through outlet G² to a light- sands tank J.” In the drawing, A¹, A², A³, A4, A5, and A6 are mixing vessels; G and K are classifiers; E is a tank containing oil emulsion. He refers to the quantity of oil several times in vague terms, explaining, however, that it should be “insufficient to materially lessen the specific gravity of the metalliferous mineral particles. ” Finally, he specifies the proportion as “usually an amount of oil varying from 4% to 6% of the weight of metalliferous mineral matter present in the ore.” This can be interpreted variously; if it refers to the sulphides to be concentrated, then an ore containing 20% blende would require from 0.8 to 1.2% of oil, equivalent to from 16 to 24 lb. oil per ton of ore. On the other hand a 2% chalcocite ore would need only 1.6 to 2.4 lb. of oil per ton of ore, which is as little as is now used.
Cattermole Flotation Process
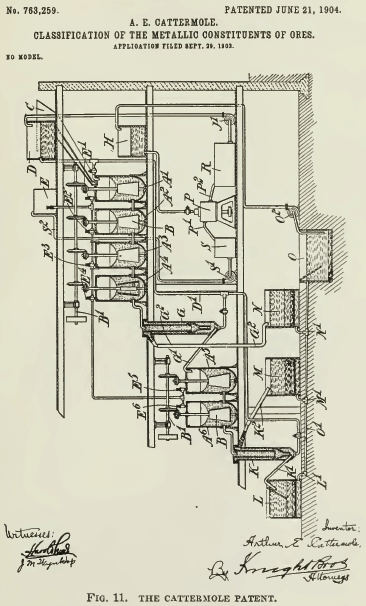 This Cattermole process was the subject of lengthy experiment in the London laboratory of the Minerals Separation company, where all sorts of variations in temperature, acidification, oiling, and mixing were tried by Arthur H. Higgins under directions from H. L. Sulman and H. F. K. Picard. It was not until March 1905, that is, nearly 2½ years subsequent to the patenting of the Cattermole method, that it was found advisable to float the ‘granules’ rather than sink them, whereupon ensued “the startling discovery of the agitation-froth process,” as W. H. Ballantyne has described it. A similar discovery was made contemporaneously at Broken Hill, but there, according to James Hebbard, it was not so “startling;” it was the result of strenuous efforts to make a workable process out of the impracticable method devised by Cattermole. See Fig. 12 and 14.
This Cattermole process was the subject of lengthy experiment in the London laboratory of the Minerals Separation company, where all sorts of variations in temperature, acidification, oiling, and mixing were tried by Arthur H. Higgins under directions from H. L. Sulman and H. F. K. Picard. It was not until March 1905, that is, nearly 2½ years subsequent to the patenting of the Cattermole method, that it was found advisable to float the ‘granules’ rather than sink them, whereupon ensued “the startling discovery of the agitation-froth process,” as W. H. Ballantyne has described it. A similar discovery was made contemporaneously at Broken Hill, but there, according to James Hebbard, it was not so “startling;” it was the result of strenuous efforts to make a workable process out of the impracticable method devised by Cattermole. See Fig. 12 and 14.
This ‘discovery’ led to Minerals Separation’s basic patent U. S. No. 835,120, of May 29, 1905, which duplicated the British patent No. 7803 of April 12, 1905, taken out in the names of H. L. Sulman, H. F. K. Picard, and John Ballot. In this patent the aid of chemically- generated gas is discarded definitely, in favor of air-bubbles. This seems to me a matter of far greater importance than the reduction in the proportion of oil. The patentees say: “It is to be understood that the object of using acid in the pulp according to this invention is not to bring about the generation of gas for the purpose of flotation thereby, and the proportion of acid used is insufficient to cause chemical action in the metalliferous minerals present.” This differentiates the method from those of Potter, Delprat, Froment, and De Bavay, the addition of acid being therefore presumably to assist the selective oiling of the sulphides. The patentees also state that “a large proportion of the mineral present rises to the surface in the form of a froth or scum which has derived its power of flotation mainly from the inclusion of air-bubbles introduced into the mass by the agitation, such bubbles or air-films adhering only to the mineral particles which are coated with oleic acid.” The last clause had better have been omitted, for it is only conjecture, as to the truth of which there is room for plenty of doubt, but the clear description of air as the main agent of flotation is most important—far more important as regards the rationale of the process, than the diminution in the proportion of oil.
As to this, it is stated that if the proportion of oil mentioned in the previous Cattermole patents “be considerably reduced—say to a fraction of 1% on the ore—granulation ceases to take place, and after vigorous agitation there is a tendency for a part of the oil- coated metalliferous matter to rise to the surface of the pulp in the form of a froth or scum.” One per cent on the ore is equal to 20 lb. of oil per ton; a ‘fraction’ of 1% is anything between 20 and 0 pounds per ton.
Chief Minerals Separation Process
 In enforcing the right to the collection of royalties, the Minerals Separation company has rested its claim mainly on the reduction of oil, claiming that it produces a series of phenomena quite different from any of the other methods employing larger proportions of oil, and, concurrently, insisting that such superior effects as are produced by the use of the reduced quantity of oil are un-obtainable when the larger proportions of oil are used. Thereupon, of course, it has been claimed, by those desiring to ignore the Minerals Separation basic patent, that neither the Cattermole nor the Froment methods demanded a quantity of oil notably larger, for the minima prescribed by these earlier inventors come under 20 lb. of oil per ton of ore. However, this matter is still sub judice, so it is best let alone for the present.
In enforcing the right to the collection of royalties, the Minerals Separation company has rested its claim mainly on the reduction of oil, claiming that it produces a series of phenomena quite different from any of the other methods employing larger proportions of oil, and, concurrently, insisting that such superior effects as are produced by the use of the reduced quantity of oil are un-obtainable when the larger proportions of oil are used. Thereupon, of course, it has been claimed, by those desiring to ignore the Minerals Separation basic patent, that neither the Cattermole nor the Froment methods demanded a quantity of oil notably larger, for the minima prescribed by these earlier inventors come under 20 lb. of oil per ton of ore. However, this matter is still sub judice, so it is best let alone for the present.
Between the Froment patent of 1902 and the Sulman-Picard Ballot patent of 1905 comes the Kirby patent U. S. No. 809,959 of December 14, 1903, granted on January 16, 1906. This is interesting as specifying gentle agitation and the use of a gas, making it possible to use thin oils instead of the viscous oils of the prior (Elmore) art. The claim is made that “the injection of gas, preferably air, into the mass, assists in the flotation of the hydrocarbon-coated particles.” The mention of air, as an assistant flotative agent, is more important than the reference to the kind of oil.
The actual part played by the oil has been misapprehended from the very first, the earlier investigators using not enough to produce bulk-oil flotation, while the later metallurgists have employed much more than was needed for bubble-levitation. The relative importance of the part played by air was persistently ignored until a late date and even then it was under-estimated. It is interesting to note that the two first patents in which air was specified as a gas suitable for flotative effects were those of F. E. Elmore and the firm of Sulman & Picard. Francis E. Elmore obtained a British patent for his vacuum-oil method under date of August 16, 1904, and duplicated it in the United States as No. 826,411 of July 10, 1905. Sulman & Picard obtained a British patent for their perforated-coil patent under date of September 22, 1903, duplicating it in the United States as No. 793,808 of October 5, 1903.
The Sulman & Picard patent just mentioned has been claimed by the Miami Copper Company as the one covering their operations with the Callow pneumatic cell. In No. 793,808 the inventors “utilize the power which is possessed by films or bubbles of air or other gas of attaching themselves to solid particles moistened by oil or the like.” They also state that they add oil “in quantity insufficient to raise the oiled mineral by virtue of the flotation power of the oil alone. A suitable gas is generated in or introduced into the mixture, such as air, carbonic-acid gas, sulphuretted hydrogen, or the like. For example, bicarbonates or carbonates, either soluble or insoluble in water (preferably the latter) or easily decomposable sulphides and the like may be used with acid solution.” Thus they lessen the emphasis on air as the prime agent.
Apparatus for separating metallic particles in ore
 The description also refers to the oiled metalliferous particles as “attaching to themselves, with a greater comparative strength than the gangue particles, the films or bubbles of gas which exist in the mass and are thus raised to the surface of the liquor by gaseous flotation.” Yet we are told that the metallurgists who prepared this excellent description of the bubble-levitation method made “a startling discovery” of the frothing process eighteen months later. This U. S. patent 793.808 is more than a year junior to Froment’s British patent, and contains an echo of it in the introductory announcement.
The description also refers to the oiled metalliferous particles as “attaching to themselves, with a greater comparative strength than the gangue particles, the films or bubbles of gas which exist in the mass and are thus raised to the surface of the liquor by gaseous flotation.” Yet we are told that the metallurgists who prepared this excellent description of the bubble-levitation method made “a startling discovery” of the frothing process eighteen months later. This U. S. patent 793.808 is more than a year junior to Froment’s British patent, and contains an echo of it in the introductory announcement.
Elmore’s vacuum-oil process marked another inadvertent step toward the recognition of air as the most important flotative agent. He utilizes the air naturally dissolved in water, releasing it for his purpose under a vacuum. The patent states that “under a vacuum or partial vacuum, air or gases dissolved in the milling water are liberated. These liberated gases may be augmented by the generation of gases in the pulp, or by introduction from an external source.” Elmore invented a most ingenious machine for his purpose. In so far as he depended upon the air in a pulp that had undergone mixing with a quantity of oil relatively small (as compared with his bulk-oil method) he furnished a notable metallurgic sign-post, but it is necessary to remember that he mixed his crushed ore in acidulated water and that the acid would cause the generation of carbon-dioxide gas, thus explaining his reference to “air or gases.”
The first inventor to break away from the use of either acid or oil and to make a clear claim for air as his sole flotative agent is Dudley H. Norris, in U. S. No. 864,856, under date of November 19, 1907, also in No. 873,586, of December 10, 1907. See Fig. 13. In his first patent he described a method for “introducing water containing air in solution into the lower end of an open-ended receptacle into which is introduced a flowing mixture of pulverized ore mixed with oil and water, thereby exposing said mixture to the continuous action of infinitesimally small nascent bubbles of air.” He does not specify the use of acid and he distinctly says that he does not wish it to be understood that his method “is limited to the use of oil, as the method can be practised successfully without mixing oil with the pulverized ore and water.” Incidentally, his method is worthy of friendly interest, for he has declared his intention to render the use of it free of tonnage royalty.
Having got rid of acid and oil, we have now reached the point where modified water mixed with the crushed ore in the presence of air suffices to form bubbles sufficiently lasting to buoy the metallic particles to the surface of the liquid.
THE PSYCHOLOGY
A potent factor in the history of flotation has been the psychology of the persons concerned in the invention, improvement, and application of the process. To understand the scanty and contradictory literature of the subject it is necessary to read between the lines with some knowledge of the personal equations that have rendered the problem so confusing to the later student. For example, it is an interesting fact that the first workable method was that of the Elmores, who, however, simply carried forward the ineffective research of Robson and Crowder, at the Glasdir mine. The Elmores knew of the experiments made by Robson, more particularly, for his simple apparatus was left on view at the mine when Francis Elmore and his brother came there in 1897 and became interested in the problem. Next there is the fact that in 1902 the Elmores placed their Australian rights to the bulk-oil process under option to the group headed by Messrs. Webster and Ballot, by whom Messrs. Sulman and Picard were employed. The latter were given every facility for becoming completely familiar with the operations of the Elmore process, but the Australian option was not exercised, and the group that had rejected the option formed the Minerals Separation company and proceeded to exploit another method themselves. Whereupon, not unnaturally, there arose charges and counter-charges of bad faith, provoking the lawsuit of 1907, which decided nothing, but left a lot of ill feeling in its wake.
The atmosphere amid which the various processes were tried at Broken Hill is illustrated by the fact that in the course of a successful, but misleading, test of the Potter method, the workmen in the Zinc Corporation’s plant made it a practice to add lubricating oil to the hot-acid solution in order to improve the result. This fact was not ascertained until several years after the test had been finished. At that time feeling ran high between the various process companies and “the Zinc Corporation’s experimental work was subjected to many unfavorable arguments of an extremely substantial nature, such as a varied assortment of scrap-iron, dropped into agitators, gearing, or pump-sumps.”
In order to understand the later developments, it should be stated that Theodore J. Hoover joined Minerals Separation Ltd. as technical adviser and general manager in October 1906 and resigned in December 1910. Unpleasant misunderstandings ensued. The first edition of Mr. Hoover’s book appeared two years later, in December 1912. James M. Hyde was in the employ of the Mexican syndicate organized by Minerals Separation for one year, from January 1910 to January 1911. At the instance of Herbert C. Hoover he went to Montana on an inspection of the Butte & Superior mine. Mr. Hoover withdrew from this business, but Mr. Hyde proceeded to test the ore and erect a trial flotation plant, in disregard of the Minerals Separation patents. Suit was brought against him by Minerals Separation in October 1911. E. H. Nutter was engaged by T. J. Hoover for Minerals Separation in 1910; he has been in the United States for that company since 1911, most of the time as its representative in San Francisco.
J. M. Callow’s American patent for his pneumatic launder is No. 1,104.755 of July 21. 1914. It covers the same idea as appears in T. J. Hoover’s British patent No. 10,929 of 1910. I am informed that Mr. Callow was unaware of Mr. Hoover’s invention, which was not patented in this country and is now the property of the Minerals Separation company. However, priority of invention as regards this apparatus is a matter of academic interest only. R. S. Towne used the same idea earlier than Mr. Callow in the form of a carborundum wheel, the central hole of which he plugged, so that the wheel served as a porous bottom.
No American application of the bubble-levitation phase of flotation to the concentration of ore was made until long after the Minerals Separation’s basic patent had been registered. As previously related, the early trials were made at Broken Hill. The first introduction of this method in the United States occurred six years after the date of patent No. 835,120. It is claimed by Minerals Separation that “if the directions of the patent are followed, the operation of the process is inevitable,” yet many years of trial and experimentation were required before flotation was used successfully in this country. The Utah Copper and the other Jackling companies made successful application of the process by aid of their own research and persistent effort. Up to 1911 the Minerals Separation metallurgists thought chalcocite could not be treated by flotation, and said so. In Mr. Hyde’s report of January 8, 1911, given as an exhibit by Minerals Separation in their suit against Hyde, it is stated that the tests carried out in the company’s London laboratory proved that “the copper ores of a good part of the Southwest and also of at least a portion of the Utah region contain chalcocite, which is not floatable by any of the methods so far tested.” This opinion epitomizes the experience gained up to that time in the London laboratory. Even in the 1914 edition of his book, Mr. Hoover mentions the presence of bornite and clialcocite as likely to limit the successful operation of the process. It is now recognized that chalcocite is easier to float than pyrite.
Hoover ore concentration apparatus

It is fair to add that, at a later date, one or two important copper companies obtained an increased revenue thanks to the insistence of Minerals Separation in recommending the use of their method. This insistence resulted in valuable contracts.
It is well to warn the reader against the inferences attempted to be made from experiments in court, or elsewhere, intended to prove that sundry effects can be obtained or cannot be obtained by following the descriptions in various patents. As a matter of fact the results depend largely upon the manipulation, performed usually by an operator who knows a great deal that was not known at the time the description was written. Moreover, the improvement in apparatus enables a later- day operator to apply recent knowledge in the course of an experiment supposedly based upon an old method. By aid of Herodotust and a slide machine it is possible to produce a performance that might well perplex a philosopher, or a judge.
In Mr. Hoover’s book the average royalty levied by the process-mongers is given as 1 shilling or 25 cents per ton. “Writing in July 1912, this author stated that the combined capital of all the companies controlling flotation processes was about $5,000,000. As the companies had then been in existence for 7 years, they should have treated 38,000,000 tons in order to return their capital and 10% per annum. Up to that time they had treated, he says, only 8,000,000 tons. Thus he drew a melancholy picture. But the adoption of the process by the big copper mining companies in this country is going to make those figures of four years ago look small indeed. This year 20,000,000 tons will be treated in the United States alone; next year, the tonnage may well increase to 30,000,000, taking no count whatever of the operations in Australia, Chile, British Columbia, Korea, Mexico, and other parts of the world. Soon it will be 100,000 tons per day in the United States alone. The process-mongers have a prize worthy of a big fight and the users have an incentive to curb any attempt to impose an excessive royalty accompanied by an embargo upon knowledge. That is where Minerals Separation has antagonized so many. They have claimed royalties where previously they had reported that the ore was unsuitable to flotation. Some of the companies that are now operating successfully went first to Minerals Separation for guidance and obtained so little assistance that they had to solve their own difficulties for themselves. One or two big companies have won reasonable terms; for instance, it has been disclosed that the Anaconda and Inspiration companies have guaranteed that if the Supreme Court reverses the Hyde case, or if they do not exercise their option to surrender their license in case of affirmance, they will treat 25,000,000 tons of ore by the Minerals Separation method by 1923, and will pay the agreed royalty thereon, this royalty being 2/3 cent per pound of copper, but not less than 12 cents per ton of ore; and meanwhile, whatever the decision of the Supreme Court, they have agreed to pay a royalty of $300,000 to Minerals Separation within a year from date—nine months ago. Having regard to the fact that the extraction by flotation, as compared with ordinary water concentration has been improved from 63 to 95%, at no greater cost in plant or of operation, it is obvious that the copper companies can well afford to pay such a royalty, if the improvement is due to the use of patents owned by Minerals Separation. That point the Supreme Court will decide at an early date.
But, as I have said, it is not the amount of the royalty but the method of levying tax and the attempt to place an embargo on all information concerning the technique of the process that has aroused opposition. The type of contract made with licensees has caused many operators to refuse to come to terms; but the more objectionable practice has been the enforcement of binding contracts on the metallurgists employed by the licensees, such contracts being legally invalid and representing an attempt to bluff the profession.
For the most part, until quite recently, the information available on the flotation process has come from patentees, their friends, and their enemies; a good many of the facts available have been elicited in the course of litigation, which has now been in progress for ten years; therefore a vast amount of non-science has been mixed with the little science that has survived amid thoroughly uncongenial surroundings. Anybody familiar with the bitter business feuds and personal vendettas generated during the course of quarrels over patent rights needs not to be told that keen prejudice, amounting in some cases to malice, has been injected into the ragged literature of flotation. The warping of scientific vision is astounding to the detached observer. Much that has got into print and more that has escaped a permanent record has been written with a jaundiced eye on the law- courts. On top of this the metallurgy of the subject has been placed under an embargo of secrecy by the owners of the chief patents, and this has been effective to the extent of preventing the technical men in the employ of the process-mongers from contributing to current knowledge. Only recently has there been any considerable contribution from independent sources of information.
Another important element in retarding the technology of the process is the ignoring of the fact that it depends far more on physical than on chemical considerations. To the physicist, not the chemist, we must look for guidance. The metallurgist hitherto has depended upon chemistry to guide him; he must now go back to school and acquire something more than a smattering of physics, if he expects to understand the problems of the new process. To most of us chemistry comes more easily because it has a sign language, that of the formula, to convey ideas, while physics depends upon the use of terms, half of which beg the question. Hence the student must begin by rejecting the use of terms that he does not understand, and when he has learned to understand them he must take pains to define them whenever he undertakes to convey his ideas to others. By such sincerity of thought it will be possible to make real progress, and to apply science to industry with results far transcending any hitherto achieved in this field of human activity.
https://archive.org/stream/flotationproces00unkngoog#page/n66/mode/2up
