Table of Contents
Unusual Applications Of The Flotation Process: In the mineral industry flotation is often defined as a physicochemical process that has the capability of separating two or more finely divided minerals from each other. In the usual concept of flotation all three forms of matter are involved and many of the physical and chemical properties of gases, liquids and solids can influence the process.
Examples of simple flotation operations might include the flotation of galena (PbS) from limestone or molybdenite (MoS2) from quartz. Progressing to more difficult separations would include mention of fluorspar, barite, galena, and quartz separations, the separation of metal- silicate minerals and the newly developed flotation processes for the selective recovery of beryllium minerals from a host of gangue minerals.
The reader of this paper should not completely accept any of the common definitions of flotation of unusual separations which now appear possible by proper application of froth flotation, foam flotation, skin flotation or agglomeration flotation. Some of the more unusual applications of flotation from solutions (even gold from sea water), selective separation of micro-organisms, selective separation of colloidal particles, and the flotation treatment of a host of organic and inorganic industrial wastes to concentrate various components.
This more liberalized concept of the very broad range of separation operations made possible by flotation has no room for the old theory that the flotation process can be applied only to particles in the range of minus 35 mesh (420 microns) to 10 microns. The volume of commercial plant production of materials beneficiated by the more sophisticated flotation methods is still very small if we compare this output to copper, lead or zinc mineral flotation. The volume of the future production of materials processed by foam flotation, agglomeration flotation, and new applications of froth flotation is limited only by the imagination and initiative of the pioneers active in this field.
Flotation Froth Functions and Characteristics
The function of flotation froths or bubbles does not vary greatly whether the type of flotation be agglomeration, froth or foam flotation. The bubbles are expected to function, in addition to other tasks, as a transport medium. The method in which the transport is carried out will vary but particle or ion movement in a planned direction is always important.
Characteristics of flotation froths or bubbles can vary from the relatively stable air bubbles generated for agglomeration and froth flotation to the less stable watery type bubble preferred in foam fractionation and ion flotation.
Clark reports studies on the use of electrical conductivity to measure the expansion factor of foam. Expansion factor is defined as the ratio of foam volume

Microscopic studies of flotation bubbles reveal many interesting facets on how bubbles “float” their load. Small bubbles present a greater total surface area and therefore provide a greater lifting force. Stable and brittle froth characteristics also receive study in relationship to the type of material being concentrated. (The two magnified photos at left show 100 mesh galena being floated and were taken by H. Rush Spedden. Photo on the right is courtesy Hercules Powder Co.) 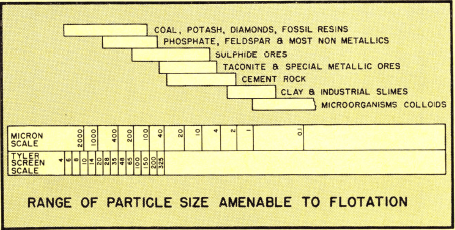 to the volume of contained liquid. As ion flotation and foam fractionation developments continue, automatic measurement of the expansion factor of foams may become important. Application of conductivity and radioactivity measuring devices should be effective.
to the volume of contained liquid. As ion flotation and foam fractionation developments continue, automatic measurement of the expansion factor of foams may become important. Application of conductivity and radioactivity measuring devices should be effective.
The Office of Naval Research sponsored some fundamental research on foam stability about a decade ago. This study showed that surface viscosity of detergent solutions was an important factor in foam stability. Foams of high stability were produced from solutions showing appreciable surface viscosity; solutions yielding foams of very poor stability showed very low surface viscosity. Also it was postulated that high foam stability is generally the result of the presence of two or more compounds, one of which has high solubility and provides a reservoir of surface active material of poorly developed coherence. The other compound, which may be present in much lesser quantity, is less soluble and provides the necessary coherence in the mixed surface film. Another variable studied was the transfer of air through adsorbed surface films as a factor in foam stability. High foam stability depends on the absorbed surface film having low permeability to air.
Researching of foams, froths, films and emulsions is being carried out in many areas of chemistry and minerals processing. The mechanism of how a soap, or any other film thins is under study. Work supported by a grant from the Petroleum Research Fund of the American Chemical Society has resulted in another new theory as to how soap film thins. The Mysels theory claims that “film thinning results when thinner film segments replace thick film segments near the film border. This means that a new surface is generated at the margins of the film. This replacement is caused by the curvature of the film surface at its edges. Freshly drawn segments are thinner than those they replace, because on their way from the edge into the body of the film, they go through a pressure drop which acts as a ‘wringer’.”
The foaming properties of nonionic detergents relate to the proper functioning of these materials in your home laundry. Researchers Shick and Beyer reported on the theory of foam formation by nonionic detergents at the 34th National Colloid Symposium at Lehigh University in 1960. In general, the nonionic syndets form unstable foams. Foam stability passes through a maximum at a point that Drs. Shick and Beyer call the critical hydrophilic-hydrophobic balance (CHHB). Foam stability is linked to two principal factors—foam drainage and film rupture. Typical nonionic detergents form fast draining foams. The foam is most stable at the CHHB where it drains the least.
Studies of foam variables such as those reviewed here have provided a foundation of knowledge from which is evolving ion flotation and foam fractionation processes. The tailoring of froth characteristics to fit a given flotation goal involves a delicate balance of many factors.
Foam Fractionation and Ion Flotation
The date of the real beginning of foam fractionation is not clearly known. The work of F. Schutz published in 1937 indicates an early discovery of the principles involved. Schutz showed that hemoglobin could be separated from serum by foam fractionation. In 1949, Schutz published information on the methods and principles of fractionation by adsorption and crystallization on foam.
A paper by Kevorkian and Gaden, Jr. in 1957 introduced the route to the Foamet Process while Dr. Sebba’s work in South Africa resulted in the ion flotation process. According to a Wall Street Journal release in 1959, Armour and Company have assisted in patenting the use of ion flotation for processing sea-water with coverage in the United States and 31 other nations.
Both foam separation and ion flotation take advantage of the fact that, in a liquid mixture, surface-active components are preferentially adsorbed at gas – liquid interfaces. Also, “surface – inactive” components concentrate in the bulk liquid phase. Since solutions of metal ions are not usually surface-active, the metal ions must be combined or complexed with a compound or material that does have surface-active properties. This apparently is the basis for the Sebba ion flotation process. The Sebba process claims formation of insoluble soaps with the ion to be recovered. The insoluble soaps are then recovered as a froth product.
The range of effectiveness for foam separation and ion flotation is reported to be in the range of 10-3 molar concentration to 10-9. This is in a dilution range considered too weak for application of ion exchange.
Research by AEC and by Radiation Applications, Inc. over a period of several years has been aimed at commercial application of foam separations. Radiation Applications now has a pilot plant available for application of the technique which is called the “Foamet” process. The basis for the process has long been known but never commercially applied. If material in a solution is surface-active, it will concentrate in a molecular layer at the surface of the solution. To greatly increase surface area, the solution is foamed. The selection of the foaming agent is critical and RAI has worked with more than 300 different foaming agents.
The pilot plant now used for foam separation utilizes a ten-foot vertical glass column with a 6-inch diameter. Continuous foam generation at the bottom forces the foam up the column countercurrent to the descending feed liquid. Values, or the substance to be removed, will concentrate at the bubble surfaces. The 6-inch foam column is enlarged to a 15-inch diameter at the top of the column to aid further separation. Then the foam is discharged into a centrifuge which acts as a foam breaker. The enriched foamate liquor can be discharged, or all or part of it recycled to the column. In some AEC work, three foam columns were operated in counter-current series. The process has been successfully used to remove strontium from nuclear process effluents with recovery exceeding 99 percent.
Bulk Flotation
The field of application of “non-selective” bulk flotation to a multitude of chemical and waste processing problems is expanding rapidly. The reference “non- selective” is intended to indicate flotation processes wherein a solid phase or phases are removed from a liquid phase. Whether one or many different solids are present in the pulp, all may report to the froth in bulk flotation.
In many applications of bulk flotation, a special type flotation cell is required. Often it is desirable to utilize dissolved gas in the pulp or the so-called vacuum- flotation approach.
Baum and Hurst discussed dissolved gas flotation theory and flotation cell design for municipal and industrial waste flotation at Purdue University in 1953. They recommended cell depths of 4 to 6 feet and cell lengths of two to three times the width when flows are in excess of 50 gpm and less than 2000 gpm. Flows smaller or larger than this necessitate a different relationship. According to Baum and Hurst, applications of dissolved gas flotation systems that merit the sewage and industrial waste engineers’ evaluation are:
- Suspended solids separation, to replace or augment sedimentation.
- Colloidal particle size separation, to replace or augment rough filtration.
- Primary treatment, to economically relieve the load on fine filtration systems.
- Oil and grease separation, to attain high removal efficiencies on emlusified and dispersed fractions.
- Primary sewage or activated sludge treatment plant operation to attain solids removal and liquid super-saturation with oxygen.”
Kalinske and Evans compare the unit processes flotation and sedimentation. Their conclusions include the statement that no one treatment method is superior and specific process application can be recommended only after careful laboratory evaluation. They make the following generalizations as regards the use of settling or flotation in separating solids from liquids in the treatment of waste liquids:
- Flotation permits use of a smaller basin than when gravity settling is used.
- In most cases a flotation unit will not give as highly polished an effluent as a gravity settling unit.
- The operating costs, primarily because of the higher power costs, will be greater for a flotation process.
- A thicker sludge can always be obtained from a flotation unit, than by gravity settling.
- Where completeness of chemical reaction, density of precipitates, and adsorption of material being coagulated is aided by prolonged slurry contact, a flotation unit should not be used; a solids-contact unit is indicated for such treatment problems.”
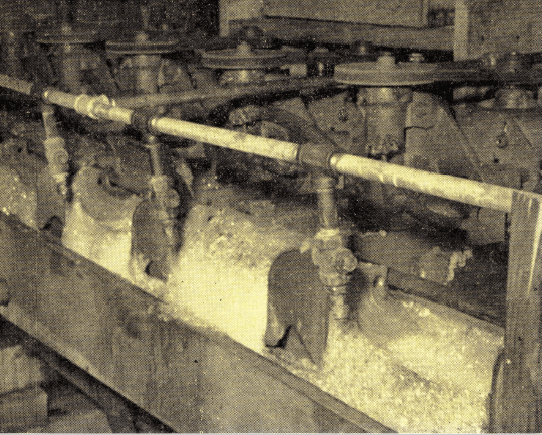 The effectiveness of a portable flotation test kit used in studies of flotation of oil refinery waste waters is reported by Rohlich. Further refinement of this equipment and method may be just the shot-in-the-arm that the industrial waste flotation industry needs. The somewhat expensive installation usually thought necessary for testing the application of froth flotation principles to waste treatment often precludes any further consideration of the process. The development of portable flotation test equipment for field use in testing industrial wastes should accelerate in the future.
The effectiveness of a portable flotation test kit used in studies of flotation of oil refinery waste waters is reported by Rohlich. Further refinement of this equipment and method may be just the shot-in-the-arm that the industrial waste flotation industry needs. The somewhat expensive installation usually thought necessary for testing the application of froth flotation principles to waste treatment often precludes any further consideration of the process. The development of portable flotation test equipment for field use in testing industrial wastes should accelerate in the future.
The use of froth flotation techniques to process waste products from atomic reactors and fuel processing wastes is growing in importance. The application of the flotation process to an atomic waste problem was reported in 1957. A uranium fuel rod ruptured and scattered radioactive flakes of material in a “room- size” heavy water container. A flotation pulp containing forty gallons of alcohol, carbonated water and two pounds of oleic acid was prepared. The radioactive flakes were attached to the bubbles and lifted out from a multiplicity of tubing which lined the bottom of the
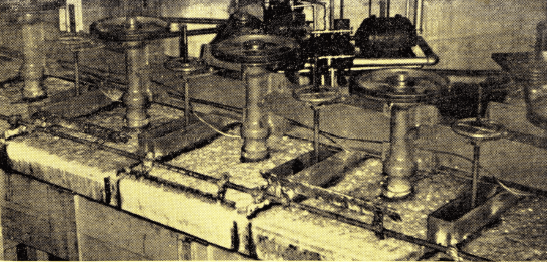
Pilot plant studies in the flotation of domestic sewage were conducted to determine effects of flotation toward the reduction of B.O.D. in sewage plant effluent.
Two 4-cell No. 30 “Sub-A” Flotation Machines handling 1,000,000 gallons per day clarifier effluent at sewage treatment plant.
container. This must be called a “non-selective” flotation process, but the desired result was obtained with a high degree of success.
The bulk flotation of oil from Canadian oil sands is an example of separation of a liquid from a solid by a froth flotation process. The removal of nonpolar oils, phenols, fatty acids, mercaptans and other organic chemicals from tank car waste solutions and slurries is an example of the separation of organic liquids from water with solid phase hydrocarbon (coal) acting as the absorbent and carrier.
Alexander MacKenzie, as early as 1789, proposed the idea of recovering oil from Canada’s famous Athabaska oil sands. Estimates made in 1950 claim that from 100 to 500 billion gallons of oil are available for recovery. The oil sands contain from 3 to 16% oil by weight.
A pilot plant, which began operation in October 1948, included froth flotation as an important concentration step. The premixed feed was heated with hot water and steam and the resulting pulp contained about 15% oil, 10 to 12% water for an average of 25% by weight of total liquids. This is the same density preferred for many common ore flotation processes in which a 70 to 75% solids pulp is conditioned before the flotation step.
At the Bitumount, Canada, pilot plant, the hot pulp was mixed in paddle mixers to break the oil film surrounding each sand grain. After the oil film is broken, a water film takes its place. The tiny oil droplets coalesce to form larger masses or drops of oil. Just before the mixed pulp was fed to the flotation cell, the oil, water and sand pulp was mixed with additional 200 degree F. recycle water. The violent agitation provided broke up any remaining lumps just before the slurry entered the flotation cells. The gently aerated flotation cells removed the oil as a buoyant froth that floated readily. The oil froth from the flotation cell contained 25 to 30% water and 4 to 8% mineral impurities such as clay and fine sand. This froth product was then refined by conventional oil refinery techniques.
In 1951 a most unusual industrial waste treatment plant began operations at Saegertown, Penn. The plant was designed for processing wastes from railroad tank car cleaning operations. Since over 700 different chemical products are transported by tank cars, it is obvious that the wastes are complex and variable. The froth flotation section of the plant is used primarily to abstract nonpolar oils as well as phenols, fatty acids, mercaptans and almost any organic chemical with an acidic functional group. Minus 35-mesh bituminous coal is used as an absorbent. Coal feed rates range from 100 to 300 pounds per thousand gallons of waste. Some pine oil is usually added to stabilize the froth and pH is usually controlled to a value of 6.0 to 7.0.
The coal and its adsorbed organic compounds are recovered as a heavy froth product from mechanically agitated flotation cells. The froth is filtered on a rotary filter and then dried and used as fuel. In this very unusual application of the froth flotation process, the mineral value (coal) is used to recover the organic compounds whereas in conventional mineral flotation, organic compounds are used to recover the minerals.
The use of dissolved air or dissolved gas flotation processes is increasing in the petroleum industry. D’Arcy, Jr. points out some of the important considerations in the selection and design of a dissolved air flotation system:
1. Dissolving the maximum amount of air in the influent.
2. Elimination of entrained air to decrease turbulence.
3. Proper hydrodynamic design of flotation system.
4. Proper selection of coagulant and floc-forming chemicals, if required.
5. Continuous mechanical removal of oil or floc from the surface of the water in the flotation chamber.
6. Include all possible automation in original design.
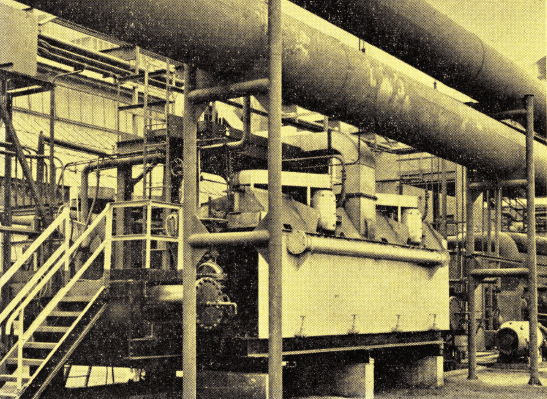
Ford Motor Company uses flotation to recover naphthalene from cooling tower water at their modern coke plant. The two 4-cell No. 30 “Sub-A” Flotation Machines each handle over 1500 gallons per minute.
Oils from the food industry that are processed by dissolved air flotation include linseed, castor, soya, cottonseed and coconut vegetable oils.
The development of processes for the treatment of laundry waste water to produce clean water for re-use has long been under study by the Navy Department. Further, a low-cost method for clarifying home laundry waste water could well be one of the laundry accessories of the future for your home. Research sponsored by the Navy resulted in a practical solution by application of the flotation process. As much as 87 to 95% of the total laundry waste water tested could be continually recovered by a flotation process.
Separation of Inorganic Materials
Many of the known mineral species are inorganic chemicals. A chemical compound may be defined as a substance composed of two or more elements combined according to the laws of chemical combination. Now the mineral formation processes do not always appear to be good “chemical-law” abiding citizens and this accounts for some of the irregularities noted in some mineral species. The flotation process has long been competitive with other unit processes for the separation and recovery of a multitude of inorganic chemicals. In some of the examples that follow, the separation noted may involve a true mineral specie, but most of the examples involve either an unusual mineral or a man- made chemical compound.
A flotation plant in Akranes, Iceland, processes a minus 28-mesh mixture of mollusk shell fragments and tuff to recover a froth product containing 90 to 95% calcium carbonate. The unusual precaution of exchanging fresh wat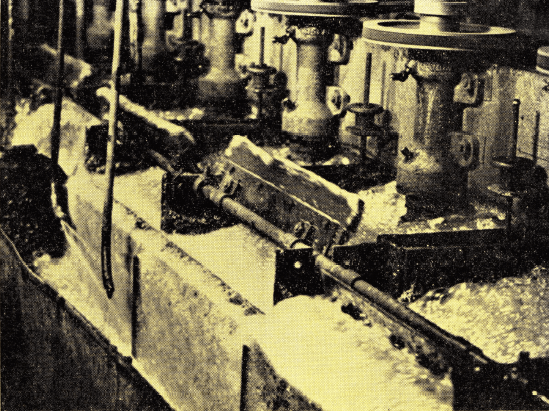 er for sea water is required before the flotation step is attempted. The waste tuff product contains only 10% calcium carbonate.
er for sea water is required before the flotation step is attempted. The waste tuff product contains only 10% calcium carbonate.
The Trans western Pipeline Company utilizes a unique sulfur flotation step in its Italian – developed Giammarco-Vetrocoke process which scrubs carbon dioxide and hydrogen sulfide from natural gas. The plant utilizing this new process is located about 35 miles from Fort Stockton, Texas, and is the first in the Western Hemisphere. In the flotation step, elemental sulfur is removed from a chemical solution by a simple aeration step in two parallel “flotation towers.” The sulfur bearing froth is filtered and the filtrate is returned to the process stream.
Elemental sulfur is floated from aragonite and gypsum gangue in an Egyptian flotation plant which uses sea water in the flotation circuit. The plant is operated by the Societe Egyptienne d’ Engrais et d’ Industries Chemiques at Ras Gemsa on the Red Sea- coast.
The recovery of sulfur dyes from waste waters of dye works has been reported. A combination of skin flotation and froth flotation is used to recover a wide range of particle sizes.
Flotation of rubber resins from milkweed was a war-time experiment and opened the door to many new inquiries of how flotation could be applied to agricultural products.
Work by Belgian engineers showed that sodium nitrate can be separated from ammonium nitrate by froth flotation. The purity of the nitrate compound recovered was as high as 94% sodium nitrate.
Flotation can be applied as an accessory process in the regeneration of spent catalyst used in the fluidized catalytic cracking of gas oil. The flotation step is carried out at a pH of 2.0 to 6.5 and fatty or naphthenic acids are used as collectors. The relatively highly contaminated catalyst particles are removed as a froth product while the less-contaminated particles report to the underflow.
Froth flotation is used in a plant near Grand Junction, Colorado to reject siliceous mineral impurities from gilsonite ore. The upgraded gilsonite ore is converted to gasoline and extremely low ash metallurgical grade coke in a refinery operated by the American Gilsonite Company. This unusual mining operation also Coal has been one of the early objects of flotation. Coal fines are recovered from the dilute washery water before it is impounded thus aiding substantially in the extensive stream pollution abatement program.
Gilsonite flotation at American Gilsonite Company incorporates “Sub-A” Flotation Cells with special froth removal rakes. Gilsonite, after being up-graded by flotation, is processed into metallurgical coke and gasoline.
utilizes hydraulic jet mining monitored by TV cameras and a 72-mile pipeline for transportation to the refinery near Grand Junction, Colorado. The delivered cost of the mineral at Grand Junction is reported to be $1.76 per ton. The 1400 ton-per-day flotation plant lowers silica content to less than 0.04%.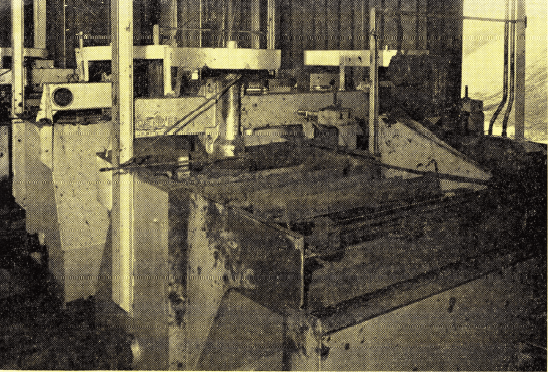
Klepetko reported details of the development of a commercial flotation process for the recovery of fossil resin from coal in 1947.
Stephens patented a process for the production of ferric oxide compositions of rouge grade which utilizes a flotation step to reduce the carbon content to less than 0.15%. The free carbon is removed as a froth product.
The separation of ferrosilicon from magnetite by froth flotation was described in a patent issued in 1952.
High-purity titanium carbide is separated from carbon and other impurities by froth flotation. The feed material particle size is substantially all minus five microns and kerosene is used as the flotation reagent. The beneficiated carbide product has sold for as much as three to five dollars per pound.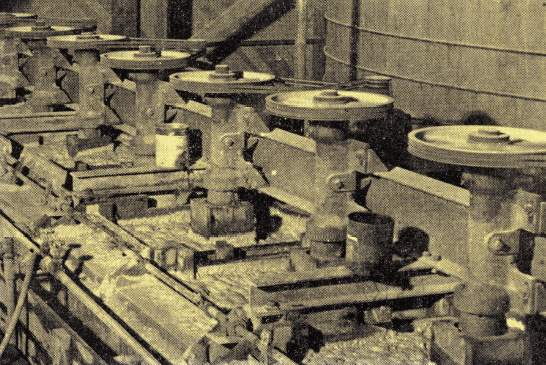
Silver compounds have long been recovered by a Sulfur flotation is conducted in these acid – proof “Sub-A” Flotation Cells. Many non-metallic and chemical applications of flotation involve acids or corrosive pulps and rubber covered parts, shaft and wood tanks are required.
flotation step in recovery systems for reclamation of values from photographic film.
Flotation research on the flotation of diamonds was reported by members of the Diamond Research Laboratory, Johannesburg, S. A., in 1951. Diamonds in their natural state may be grouped into two classes, (1) the water-repellent diamonds, which adhere to a grease surface, and (2) the water-avid diamonds, which, because of their wettability, will not adhere to a grease surface. Class (1) diamonds finer than minus 16 mesh were floated at a pH of 7 to 9 with Aerofloat reagent, cresylic acid and a frother. Class (2) diamonds are not as easy to float. Partial flotation can be achieved by preconditioning the ore with fatty acid soaps and amines followed by washing before flotation in a pneumatic cell. After the preconditioning step the same reagents used for class (1) diamonds can be used. When these less floatable diamonds are processed, the maximum particle size limit drops to 28 mesh due to poorer floatability.
According to Daily, the Consolidated African Selection Trust Limited plant at Atwatia, Ghana, uses a unique flotation process to recover diamonds. “The belt flotation machine consists of a steel trough in which there is mounted on 25-foot centers a 12-inch wide endless belt made of 80 mesh phosphor bronze woven wire screen.” In operation, a thin layer about two particles deep of diamondiferous material is deposited on the belt and carried slowly forward. As the belt dips underwater, the diamonds float on the surface of the water, often forming clusters or rafts. These clusters are carried over a weir by a current of water while the reject drops off the submerged pulley into a tank. Sometimes a small quantity of Diesel oil is added to the feed end of the belt.
Separation of Organic Materials
Many of the chemicals used as flotation reagents are of organic origin. The important phosphate rock flotation process is usually initiated by an anionic flotation step with tall oil or tall oil soap as the collector reagent. Tall oil is a by-product of the paper industry. In spite of the long history of use of organic chemicals in flotation processes as reagents, there have been relatively few applications of the flotation process to recover or separate organic materials from one another or from inorganic compounds.
Process engineers from Australia have reported on the commercial use of flotation to recover wool wax from wool scour liquors. Mechanically agitated cells are used with several froth cleaner stages. Wash water is added in the cleaner stages to further purify the wax-froth product. The concentrate contains 20% wax after draining. Final upgrading of the wax is accomplished by dispersion in an alkaline solution and centrifugal separation. The flotation process increased overall wax recovery by about 50% as compared to previous processing methods.
Froth flotation has been used to separate ink, wax and asphalt particles from waste paper.
The selective separation of various types of plastics scrap by flotation has been applied on a small scale in the relatively new industry of reclamation of plastics scrap. It requires little imagination to visualize the colorful separation process presented by the flotation of plastic materials. Some plastics require little or no reagents due to the naturally hydrophobic nature of the surfaces.
The separation of unwanted nightshade berries from shelled garden peas has been accomplished on a commercial scale by froth flotation. Coincidentally bits of skin, injured peas and other trash is floated off. Other applications in the food industry include flotation of unwanted materials from lima beans, vegetable soys and sweet corn.
A patent assigned to the U.S. Government covers the use of froth flotation to separate gluten from a crude gluten-bran mixture. The separation of a mill starch liquor into gluten-rich and starch-rich fractions by selective flotation is covered in a patent assigned to Corn Products Refining Company.
Flotation in Space
Gaudin and co-workers published results of their work carried out for the U. S. Army Chemical Corps on the separation of microorganisms by flotation. A pneumatic flotation cell of new design was developed to facilitate work on bacterial pulps which exhibited intensive frothing characteristics. Mechanically agitated sub-aeration cells were also used in some tests. The flotation cells were sterilized between tests with sodium hypochlorite solutions. The choice of flotation vessel depended upon the inherent frothing properties of the sediment being tested and upon the quantity of feed available. The pneumatic cell was utilized with feeds that frothed excessively or when large quantities of spore sediment were available. A sub-aeration cell was used for small volume samples.
As is true in some mineral flotation processes, Gaudin found that heating of the spore preparation and then cooling to room temperature prior to flotation often aided in the separation. Other factors which influenced the flotation of the microorganisms were the age of the spore material at harvest, the soluble salt content of the feed, pH of the pulp and the addition of amine or fatty acid collectors.
A most unusual method for controlling the carbide-water reaction in the generation of acetylene gas was reported by Gibadlo. The method involves the application of an off-on froth flotation process in which the calcium carbide particles are maintained in a controlled teeter between an oil phase and a water-organic phase. The oil phase floats on the water. As the carbide reacts with the water, bubbles are generated and act as flotation bubbles to lift the carbide up into the oil phase where the reaction is stopped. After the gas bubbles escape up through the oil layer to the surface, the
De-inking waste paper by flotation has been a great success and bright, high-strength newsprint made from flotation de-inked stock is used extensively in the Chicago area. “Sub-A” Flotation is the pioneer in this new field.
carbide sinks down into the water phase and reaction is reinstated.
As pointed out by Sebba, there is a possibility of using the ion flotation technique in reverse to solve one of the difficult sewage effluent problems. “The foaming nuisance of dodecylbenzene sulphonate and other detergents is a major problem of modern sewage disposal plants. The addition of aluminum ions to the solution can change the foam to an easily handled scum, and in the same way, ferrocyanide ions can be used to remove any cationic soaps that may have escaped into sewage effluents.” In May, 1961 the U. S. Public Health Service awarded a research contract to Radiation Applications, Inc. which has as its objective the removal of synthetic detergents from sewage streams by foam fractionation.
It does not require much imagination, after a review of the work of Cook and Hopper to forecast that flotation processes may play an important part in future outer space exploration. Flotation processes have the versatility to re-process drinking water and to separate algae for food production. Both of these processes are under study by laboratories concerned with outer space exploration. Thus, the astronaut, as he soars through space may well ponder his reliance on the flotation process — it produced the potash and phosphate from whence indirectly came his food pills, beryllium, nickel, molybdenum and titanium used in structural members of his ship, the copper, silver, gold and pure silica used in his communication systems, and will reclaim both food and drink from the waste products he generates during his trip to outer planets.
Flotation processing of uranium ores has resulted in upgrading and blending so that marginal grade deposits can operate at a profit.
