Table of Contents
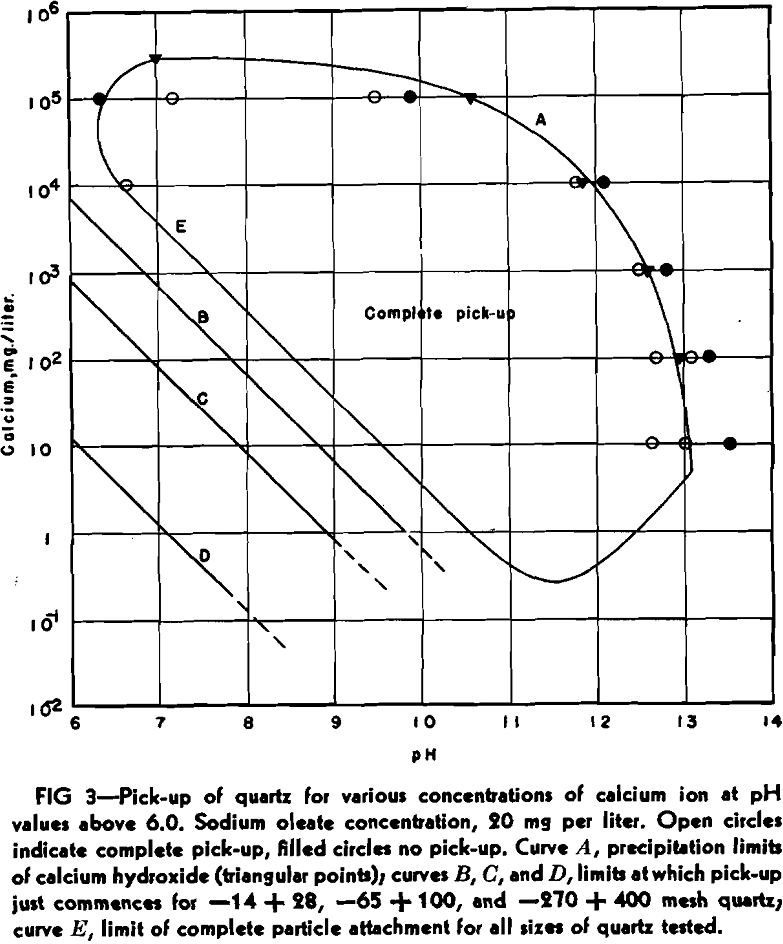
On the basis of experiments conducted on quartz using a bubble pick-up method, it was shown in an earlier paper that this mineral will preferentially adsorb hydrogen, calcium, or sodium ions, depending on the relative concentrations of those ions in the solution in which the quartz is immersed. For quartz particles ranging in size from 0.2 to 1 mm, it was demonstrated that the concentration of calcium in solution (assumed present as ions) necessary to completely activate quartz for flotation is given by the expression:
Ca++ = [H+] x 10 6 + [Na+] x 10-³
Effect of Size of Quartz on Pick-Up
To ascertain the effect of particle size on the adsorption of calcium ions, the quartz was sized from minus 14 plus 20 mesh through the intervening screen sizes to minus 270 mesh plus 400 mesh. Each size was thoroughly cleaned, and then tested in the cell at different calcium chloride and sodium hydroxide concentrations, and at a constant sodium oleate concentration of 20 mg per liter.
All particles, within the size range given, exhibited complete pick-up within the curve expressed by the equation above. This presumably means, when the conditions imposed by the equation are satisfied, that this maximum is independent of particle size.
Effect of High Alkalinity on Pick-Up
At calcium concentrations of between 1 and 10 mg per liter, and at high alkalinities, it was noticed that pick-up ceased as soon as calcium hydroxide commenced to precipitate. This effect was investigated at other calcium concentrations, with the same results. Solutions of calcium chloride, containing 10 5, 10 4, 10³, and 10² mg of calcium per liter were made alkaline with sodium hydroxide until calcium hydroxide just started to precipitate, according to the following equation:
CaCl2 + NaOH → Ca(OH)2 + 2NaCl
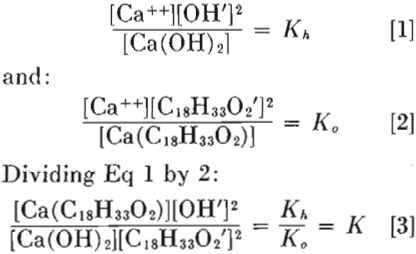
Flotation Tests
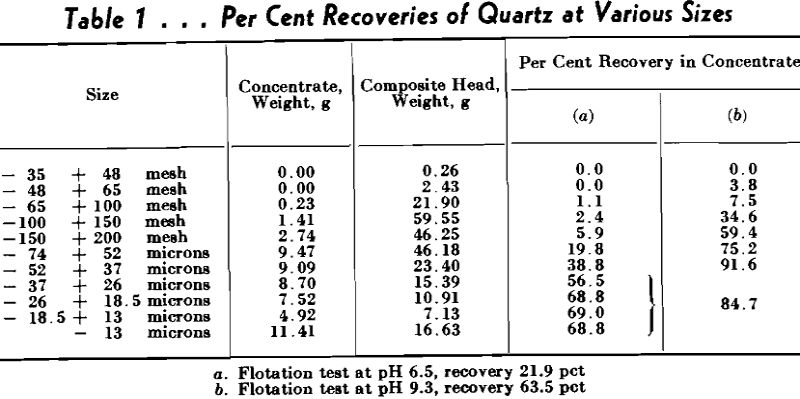
A series of flotation tests was made to ascertain the correlation between conventional flotation and the pick-up results. Because of the unavailability at the time of massive quartz, the flotation tests were made on Ottawa sand.
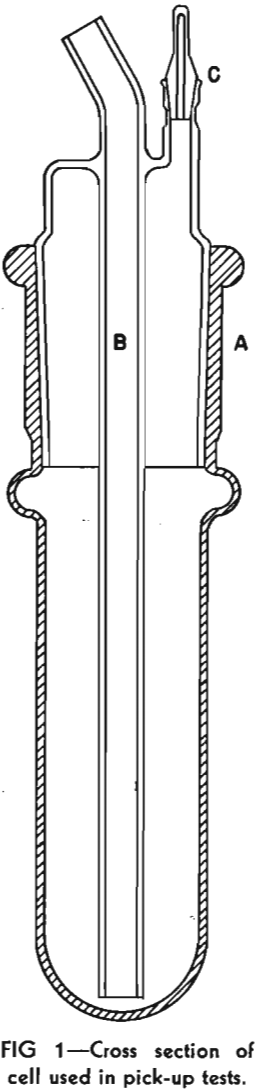
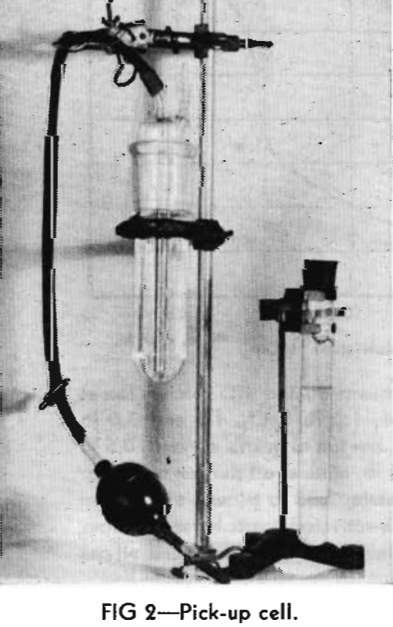
The 250 g samples of sand, with a size range of minus 28 mesh, plus 48 mesh, were boiled for 10 min with 100 ml of concentrated hydrochloric acid and 50 ml of water to leach out iron and calcium. The leached sand then was washed ten times by decantation, filtered, and washed with distilled water until the washings gave no test for chloride ion with silver nitrate. Each lot was prepared for flotation by grinding in a pebble mill for 15 min at a fixed dilution with distilled water, filtering through a Buechner funnel, washing with a series of applications of boiling distilled water, and then transferring to the flotation cell. The pH of the distilled water used in grinding was 5.6, and that of the water filtered from the grind was uniformly 6.5.
Curve A shows the recovery percent-
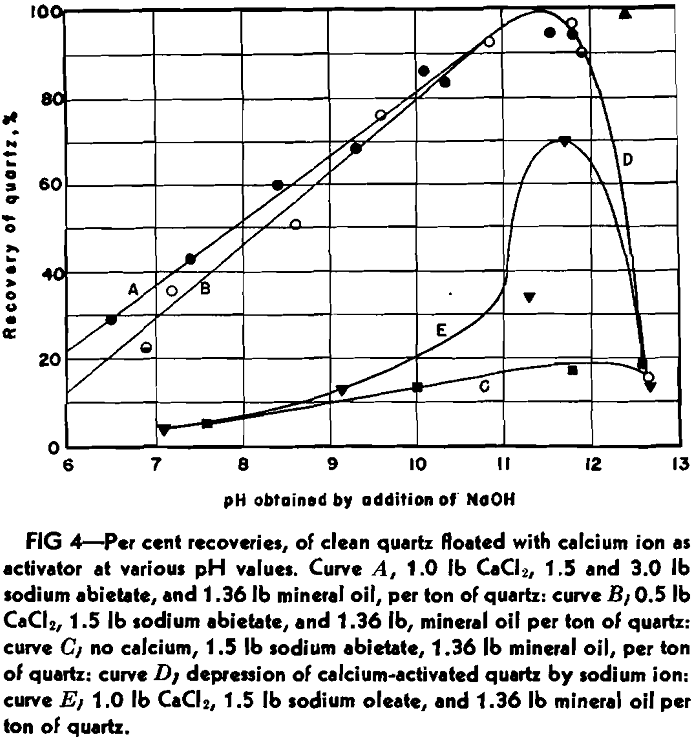
age of quartz, using a constant quantity of calcium chloride (1.0 lb CaCl2 per ton of ore, or at the dilution employed, 75 mg of calcium ion per liter of water), and both 3.0 and 1.5 lb of sodium abietate per ton of ore. Curve B represents the recoveries using 0.5 lb of calcium chloride and 1.5 lb of sodium abietate per ton. In all cases 1.36 lb of heavy white mineral oil was used.
Curve C cannot be explained by calcium activation, for the quartz was treated by acid. It is possible that ferric ion is responsible, however.