Table of Contents
The basic flotation characteristics of goethite were determined by using vacuum flotation, contact angle measurement, and Hallimond tube flotation, and the results have been correlated with information on the electrical condition existing at the goethite-aqueous solution interface. The results have also been used to interpret the batch flotation tests made on an artificial mixture of quartz and goethite. The investigation has led to the following conclusions:
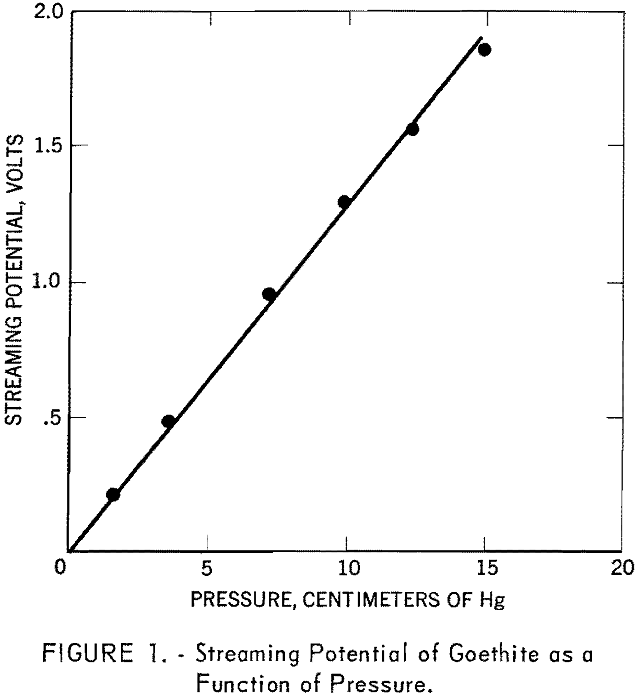
- The isoelectric point of goethite occurs at pH 6.7 and is independent of the concentration of sodium chloride in solution. The goethite surface is positively charged at pH values lower than 6.7 and negatively charged at higher pH values.
- Anionic collectors are effective on positively charged goethite, and cationic collectors are effective on negatively charged goethite. This conclusion is augmented by the intersection of the recovery versus pH curves for 10 -4 M. dodecylammonium chloride (a cationic collector) and 10 -4 M. sodium dodecyl sulfate (an anionic collector) at pH 6.7., close to the isoelectric point of goethite.
- Anionic collectors are effective on positively charged quartz, and cationic collectors are effective on negatively charged quartz. This conclu-sion is augmented by the intersection of the recovery versus pH curves for 10 -4 M. dodecylammonium chloride (a cationic collector) and 10 -4 M. of sodium dodecyl sulfate (an anionic collector) at pH 2, close to the isoelectric point of quartz.
- The critical pH curve obtained from the vacuum flotation results strongly suggests a quantitative exchange of dodecylammonium and hydrogen ions at the goethite surface. There seems to be a similar exchange between anionic collectors and hydroxyl ions at the goethite surface.
- Sodium dodecyl sulfate and sodium dodecyl sulfonate are equally effective collectors for goethite.
- Sodium dodecyl sulfate is not an effective collector for quartz at any pH value.
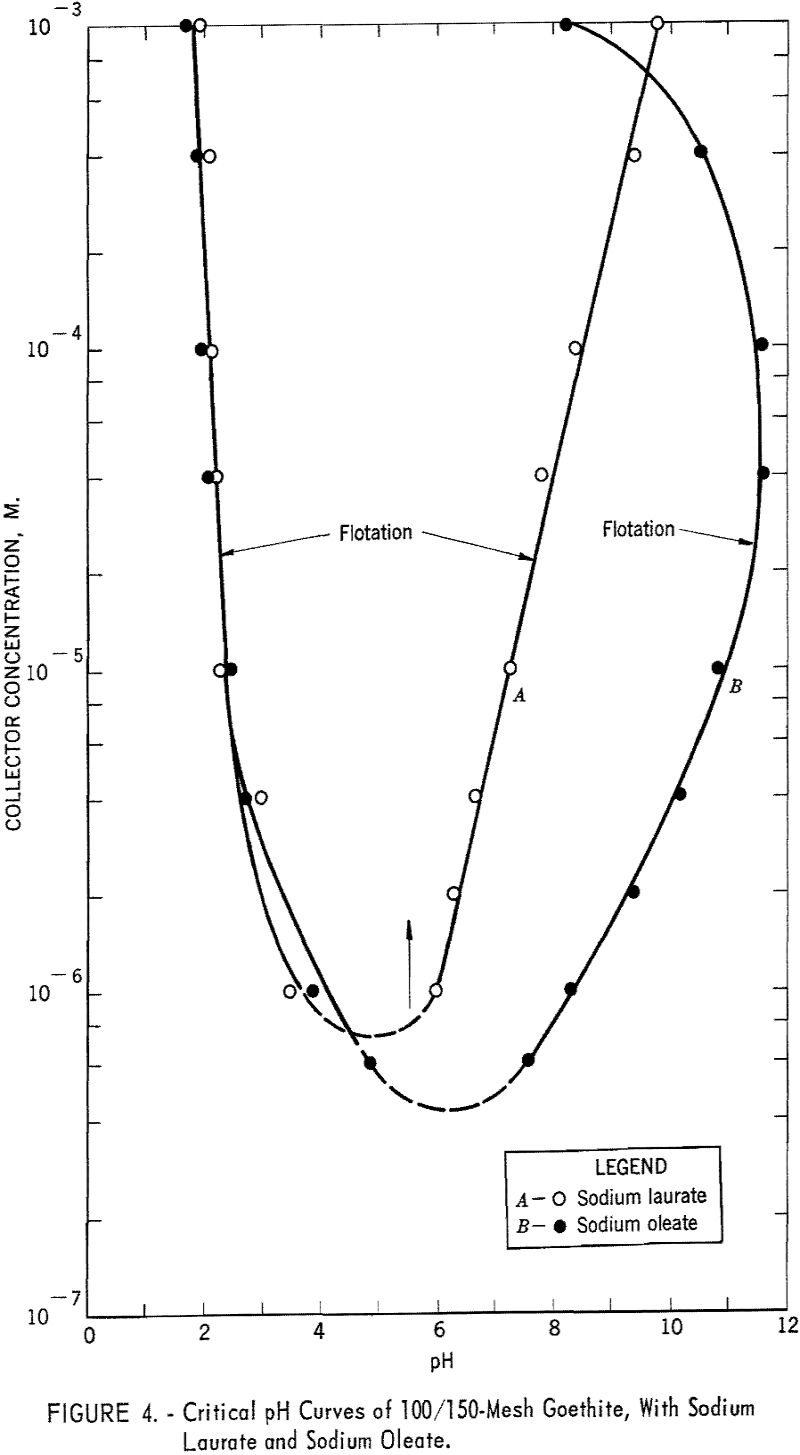
- At equivalent collector concentrations up to nearly 10 M., sodium oleate will float goethite over a broader pH range than sodium laurate.
- Optimum flotation of goethite using 10 -4 M. dodecylammonium chloride occurs from pH 8.8 to 12.0. The minimum collector concentration of this collector required for good flotation of goethite is 3 x 10 -5 M.
- Dodecylammonium chloride is a more effective collector of goethite than trimethyldodecylammonium chloride.
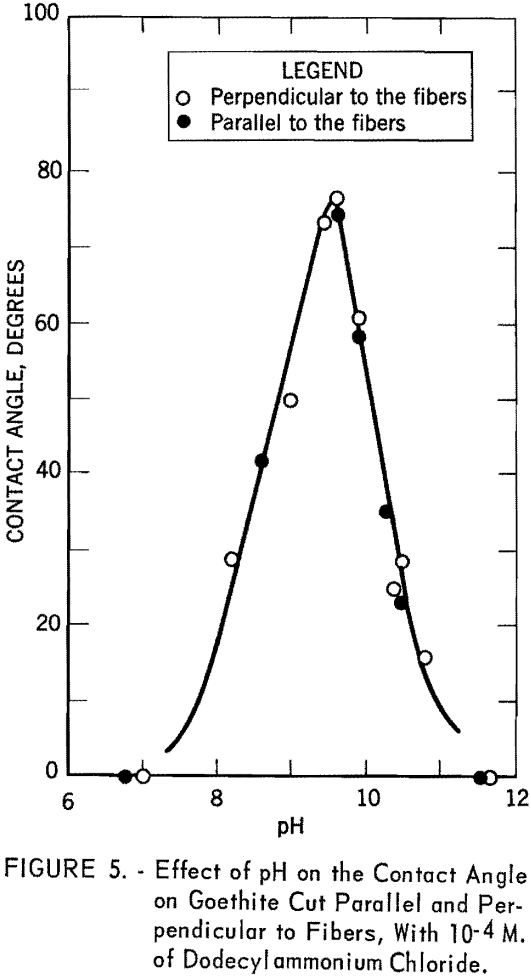
- Contact angle measurements using dodecylammonium chloride on specimens cut parallel and perpendicular to the fibers of goethite give identical results.
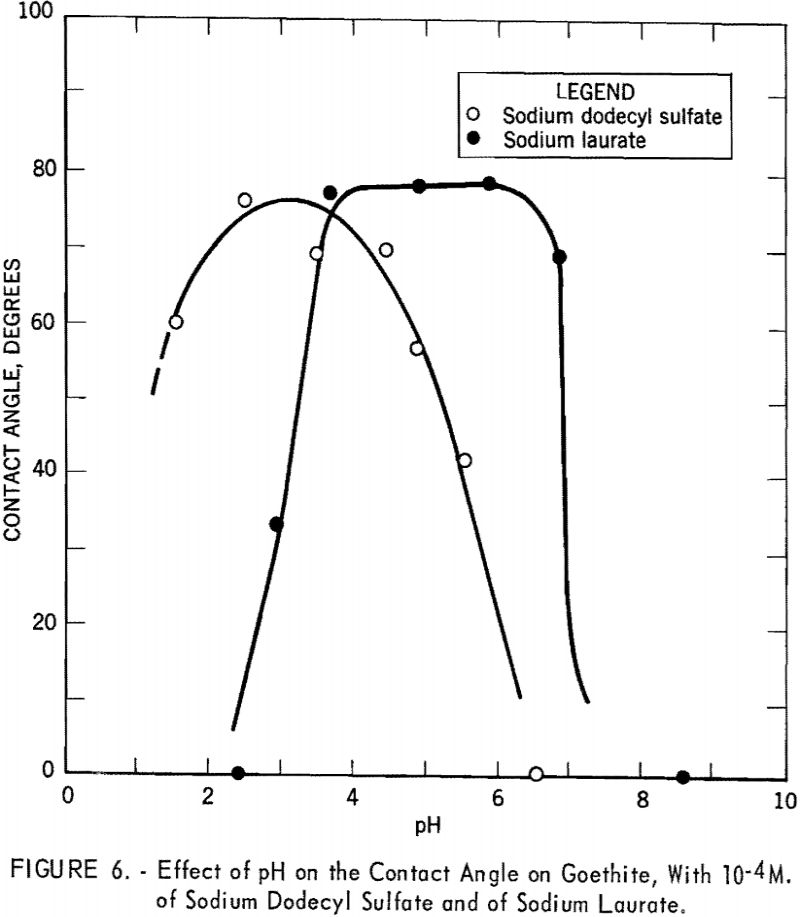
- The maximum average contact angle measured on goethite with dodecyl-ammonium chloride, with sodium laurate, and with sodium dodecyl sulfate was 77°.
- Optimum flotation of goethite from a mixture of quartz and goethite using 10 -4 M. sodium dodecyl sulfate occurs from pH 1 to 3.5.
- Optimum flotation of quartz from a mixture of quartz and goethite using 10 -4 M. dodecylammonium chloride occurs from pH 5.5 to 7.5.
- In anionic flotation of goethite from a mixture of quartz and goethite using 10 -4 M. sodium dodecyl sulfate at pH 4.2, the addition of a relatively large quantity of goethite slime (5 percent) has very little effect on the flotation of goethite.
- In cationic flotation of quartz from a mixture of quartz and goethite using 10 -4 M. dodecylammonium chloride at pH 6.4, the addition of a small quantity of goethite slimes (0.5 percent) almost completely inhibits flotation of quartz.
In recent years the rapid depletion of high-grade iron ores and the demand by blast furnace operators for increasingly low-silica ores have stimulated interest in flotation studies of low-grade iron ores, such as wash-ore tailings and oxidized taconites. Four processes have been reported for the flotation separation of these iron ores anionic and cationic collection of the iron oxides, cationic collection of siliceous gangue, and anionic collection of activated siliceous gangue. The effectiveness of any one method, however, depends largely on the mineralogical composition and on the physical structure of the sample; hence, a comprehensive study of the floatability of the individual minerals occurring in these materials is essential. A review of the literature shows that the floatability of hematite and magnetite has been frequently investigated, whereas very little corresponding information is available for goethite, or for its more earthy form, limonite, both of which are abundant in the Mesabi and Cuyuna ores.
The purpose of this investigation was to formulate general information on the floatability of goethite. Vacuum flotation, contact angle measurement, and simplified flotation tests using a modified Hallimond tube were used. The experimental results were correlated with the electrokinetic properties of goethite as determined by streaming potential measurement. The results were used to interpret the batch flotation tests made on an artificial mixture of quartz and goethite.
Preparation of Goethite Sample
Relatively pure goethite, having a radiating fibrous structure, was obtained from the Maroco mine on the Cuyuna range and used in this investigation. A hand-picked sample of about 5 kg. was first crushed in a jaw crusher and then in a rolls crusher. The product was screened at 100-mesh and a portion of the oversize was stage-ground wet in an Abbe porcelain mill until enough minus-100-mesh material was obtained. Each stage of grinding lasted 5 minutes. The dry screened undersize and the Abbe mill product were combined and wet screened at 150-mesh; the 100/150-mesh fraction was used for the flotation tests. The sample was treated with 15 percent hydrogen peroxide solution for one-half hour and then washed by decantation with demineralized water. The cleaned goethite was stored under demineralized water. Chemical analysis of the sample is presented in table 1.
For the streaming potential measurements the 48/65-mesh fraction was separated from the plus-100-mesh material. The mineral was treated in a warm solution of 1 N hydrochloric acid for one-half hour and then washed with demineralized water until there was no response to the silver nitrate test for chloride ion. The product was stored in demineralized water in a stoppered pyrex glass jar.
Goethite slime was prepared by dry grinding the 150/200-mesh cleaned fraction for 8 hours in an Abbe porcelain mill followed by pneumatic elutriation in a Haultain infrasizer. The minus-20-micron products from cones 4 through 7 were combined and used to determine the effect of slimes on flotation.
Quartz Sample
A 500-gram sample of Montana pegmatite quartz (100/150-mesh) was leached with aqua regia and washed with demineralized water by decantation until free of foreign electrolyte. The cleaned quartz was stored under demineralized water.
Periodic vacuum flotation checks were made on the goethite and the quartz samples to determine whether flotation would occur without the addition of a collector. All such tests indicated that at no time did the mineral surfaces become contaminated with organic material which would make the minerals floatable.
Reagents
The following collectors were used in this investigation:
C12H25NH3Cl – Dodecylammonium chloride
C12H25N(CH3)3Cl – Trimethyldodecylammonium chloride
C11H23COOH – Lauric acid
C17H33COOH – Oleic acid
C12H25SO4Na – Sodium dodecyl sulfate
C12H25SO3Na – Sodium dodecyl sulfonate
Dodecylammonium chloride (m.p. 28° C.), trimethyldodecylammonium chloride, and lauric acid (m.p. 44.03° C.) were obtained from Armour and Co. The water-insoluble lauric acid was converted to a water-soluble soap before use by adding 100 percent excess of the stoichiometric requirement of sodium hydroxide solution and digesting the mixture in a water bath at 55° C. until all the acid was in the soluble soap form.
Oleic acid having a reported purity of 99.8 percent was received from the Hormel Institute in an ampoule, transferred to a smaller ampoule in a nitrogen atmosphere, and stored in a refrigerator. The iodine value of the oleic acid was 89.8 (theoretical 89.87). This acid was converted to sodium oleate in the same manner as the lauric acid.
Sodium dodecyl sulfate and sodium dodecyl sulfonate were supplied by the Agricultural Research Service, U.S. Department of Agriculture, Philadelphia, Pa. The former was prepared by sulfating redistilled dodecanol with chlorosulfonic acid and the latter by recrystallization of the commercially available product.
Demineralized water containing less than 0.1 p.p.m. of salts as sodium chloride was used in the preparation of all solutions and for all test work. Reagent grade sodium hydroxide and hydrochloric acid were used as pH regulators.
Experimental Procedure and Results
Determination of the Isoelectric Point
The electrokinetic behavior of goethite in aqueous solutions was investigated by the streaming potential technique. The method consists of forcing a liquid through a porous plug of goethite particles under known applied pressure, P, and measuring the potential difference, E, generated across the plug. The zeta potential, ζ, is calculated from the streaming potential data according to the equation:

where η is the viscosity, ε the dielectric constant, and λ the specific conductance of the solution. The cell assembly was patterned after that of Fuerstenau. The streaming potential was measured with a Kintel 202B microvoltmeter. Specific conductance was determined by measuring the resistance of the plug in situ with an Electro-measurements Model 250 Impedance Bridge.
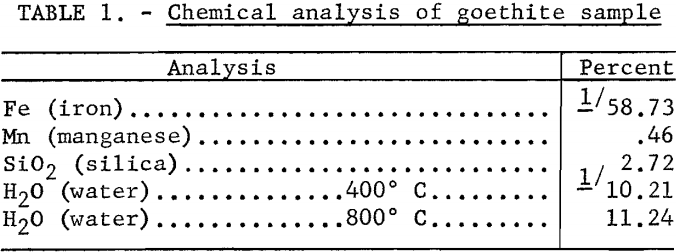
The effect of pressure on the streaming potential developed across the goethite plug was investigated in a solution of 1.2 x 10 -5 M. (moles per liter) hydrochloric acid. The results, plotted in figure 1, agree with the theory that the ratio E/P is a constant and independent of the direction of liquid flow.
The streaming potentials of the goethite in 10 -4, 10-³, and 10-² M. sodium chloride solutions at different pH values were measured and the corresponding zeta potentials calculated according to equation (1). The results are plotted in figure 2. The figure shows that all three curves cross at pH 6.7, where the zeta potential is zero. This indicates that H+ and OH- behave as potential-determining ions and fix the electrical condition at the solid-solution interface. The goethite surface is thus shown to be positively charged below pH 6.7 and negatively charged above this pH.
Vacuum Flotation Tests
A critical pH curve determines the limits of flotation of a mineral, insofar as they are governed by collector concentration and pH. Such a curve expresses flotation behavior of a mineral by defining fields of “flotation” and “nonflotation”. In the present investigation the vacuum flotation technique of Schuhmann and Prakash was used to establish the critical pH curves for goethite. That portion of the curve on the more acid side is frequently called the lower critical pH curve; conversely, that on the more alkaline side is known as the upper critical pH curve. In this paper the
lower pH curve will be called the acid limit of flotation, or simply the acid limit, and the upper critical pH curve will be known as the alkaline limit of flotation, or similarly the alkaline limit. The criterion of flotation or nonflotation was arbitrarily set at 5 to 10 percent floatability of the mineral.
The conditions for flotation of goethite with sodium dodecyl sulfate are given by curve ABCD in figure 3. In solutions more acid than about pH 1 and as defined by curve AB, goethite does not float, irrespective of the collector concentration. Slightly above pH 1, however, goethite floats at all collector concentrations in excess of 10 -6 M. until the alkaline limit is reached. This limit (CD) is linear from pH 3.8 to 7.5 at 10 -6 M. to 10-³ M., respectively, of sodium dodecyl sulfate.
Figure 3 also shows the fields of flotation for goethite with dodecyl-ammonium chloride (curves EFGHI and EFGHJ). The acid limit (EF) is linear between pH 6.3 and 8.3 with 10-³ M and 10 -5 M of the collector, respectively, and is confirmed by both vacuum flotation and Hallimond tube tests. Comparison of the alkaline limit with sodium dodecyl sulfate and the acid limit with dodecylammonium chloride shows that the anionic collector is effective in acid pulp, whereas the cationic collector is effective predominantly in alkaline pulp. The intersection of the lines showing the respective limits occurs at pH 6.7, corresponding to the isoelectric point for the mineral.
An apparent complication occurs in the fixing of the alkaline limit for the cationic collector and depends upon whether the vacuum flotation method or the Hallimond tube is used. From G, the nadir, to H a smooth curve is indicated by two points obtained with vacuum flotation and two with the Hallimond tube. From point H, with increasing collector concentration, vacuum flotation tests give a limit defined by HI. This curve coincides with the calculated free amine precipitation curve. The Hallimond tube tests give a limit defined by HJ.
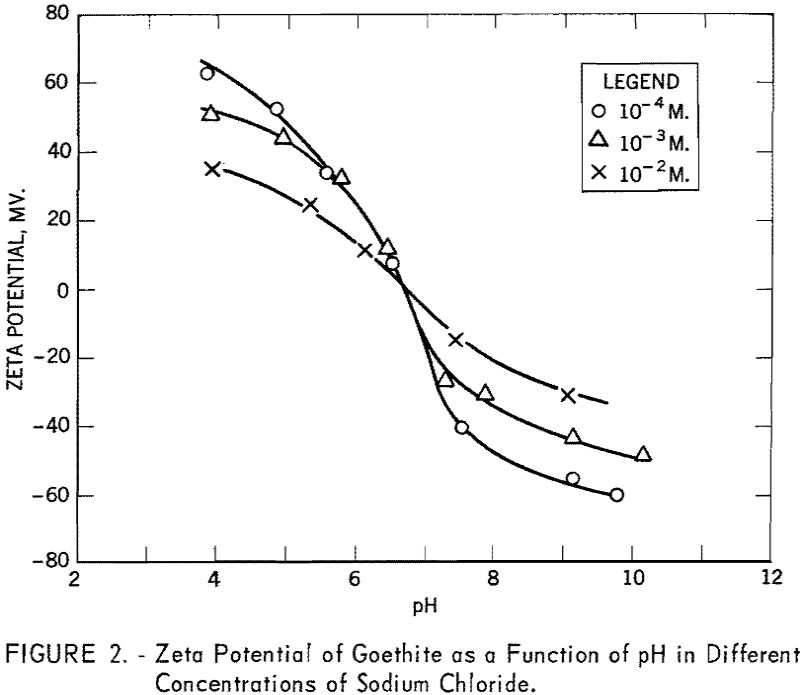
In the region enclosed by IHJ the production of voluminous froths made it impossible to use a full vacuum in the vacuum flotation tests and may explain the discrepancy. Although gas bubbles were precipitated from the solution with less than the full vacuum, there were probably insufficient bubbles precipitated on the goethite particles to give effective flotation. The precipitated gas bubbles may have been nucleated by the free amine in the system, thus causing a decrease in the number of gas bubbles available for the flotation of the goethite, The region IHJ was checked with Hallimond tube tests and it was found that for all collector concentrations above 4 x 10 -5 M. good flotation was observed for pH values up to 12.0.
Figure 4 presents the fields of flotation for goethite with sodium laurate and sodium oleate. With the former the acid limits of flotation range from pH 1.8 to 3.5 at 10-³ M. to 10 -6 M. of collector, respectively. Inasmuch as the dissociation constant for fatty acids is approximately equal to 10 -5, an increase in hydrogen ion concentration profoundly affects their dissociation. The lack of flotation below pH 2 is presumably due to the precipitation of undissociated lauric acid. The alkaline limits of flotation are defined by a linear curve ranging from pH 6.0 to pH 9.8 at 10 -6 M. to 10-³ M. sodium laurate, respectively.
With sodium oleate, goethite has a flotation range from approximately pH 2 to pH 12 for collector concentrations of 4 x 10 -5 M. and 10 -4 M respectively. The acid limits are almost identical with those for sodium laurate, but the curve showing the alkaline limits of flotation is concave toward the concentration axis. The decrease in floatability of goethite at higher concentrations of collector may be related either to the precipitation of an undetermined phase of oleic acid (presumably salted-out oleate) or to reverse orientation of collector at the mineral-solution interface.
Contact Angle Measurement
Contact angle measurements were made on two specimens of goethite, one cut perpendicular to the fibers and the other parallel to the fibers. The captive bubble technique was used for the measurements. Figure 5 shows the contact angle versus pH curve with dodecylammonium chloride at a concentration of 10 -4 M. Open circles represent the experimental points obtained for the specimen cut perpendicular to the fibers. Filled circles were obtained with the specimen cut parallel to the fibers. It is obvious that the contact angles are independent of the orientation of the mineral; hence, all subsequent contact angle measurements were made on the specimen cut perpendicular to the fibers. The curve rises rapidly from pH 6.8, where the bubble showed only a slight tendency to stick, to a maximum value of 76° at pH 9.5. It then drops abruptly to pH 11.7, where again the bubble shows only a slight tendency to stick.
Similarly, curves obtained with 10 -4 M. of sodium dodecyl sulfate and of sodium laurate as collectors are shown in figure 6. The curves are now on the acid side and show broader maxima, with contact angles of 76° at pH 2.55 with sodium dodecyl sulfate and 78° at pH 4.0 with sodium laurate.
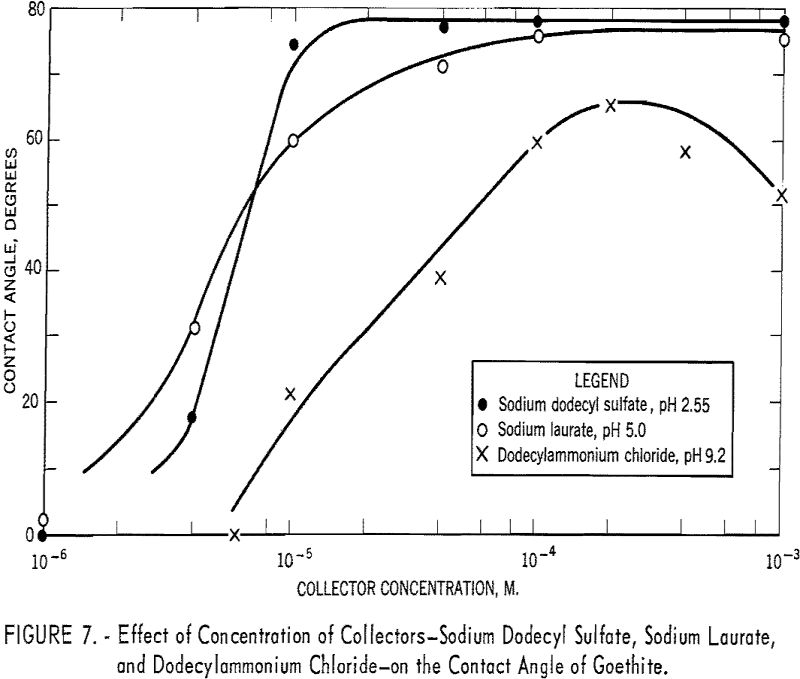
The effect of change in concentration of the three collectors on the contact angle is shown in figure 7. With dodecylammonium chloride at pH 9.2, the contact angle increases with concentration to a maximum of 65° at 2 x 10 -4 M. and then drops gradually to 52° at 10-³ M. of collector. With sodium dodecyl sulfate at pH 2.55 and sodium laurate at pH 5.0, the curves reach the maximum angles of 76° and 75°, respectively, and remain practically constant at increasing collector concentrations up to 10-³ M.
Attempts made to obtain a pH versus contact angle curve at 10 -4 M. of trimethyldodecylammonium chloride and a concentration versus contact angle curve for the same collector at pH 9.2 were unsuccessful. The bubbles that were pressed on to the surface tended to stick but rolled off when released from the bubble-holding tube. The implication is that the angles of contact were low.
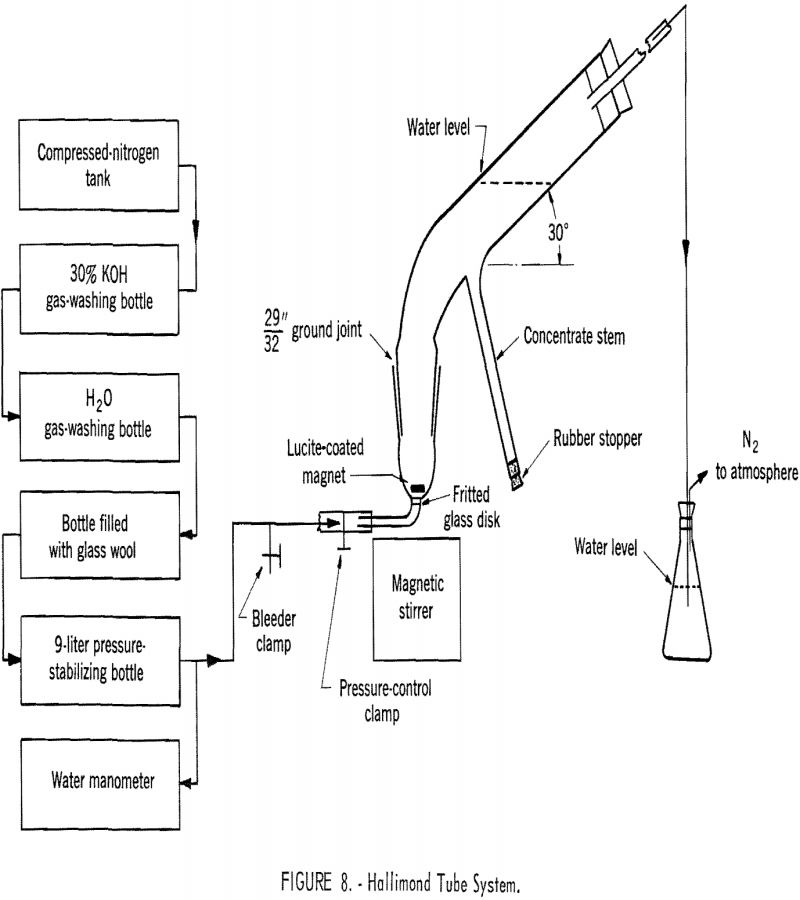
Hallimond Tube Tests
As suggested by Fuerstenau, Metzger, and Seele, quantitative study of flotation behavior on a miniature scale may conveniently be carried out with a modified Hallimond tube. In this investigation the flotation cell was modified slightly by replacing the capillary for gas introduction with a coarse-pored fritted glass disk. Figure 8 is a schematic drawing of the gas-washing system, the Hallimond tube flotation cell, and the accessory equipment. Nitrogen gas was used for aeration. After a number of preliminary tests, the Hallimond tube was standardized to operate with approximately 1 gram of the mineral sample at a gas flow of 35 ml. per minute and with a flotation time of 5 minutes. The sample was conditioned in a 100-ml. volumetric flask, filled with the test solution, by tumbling at a speed of 30 r.p.m. for 45 minutes.
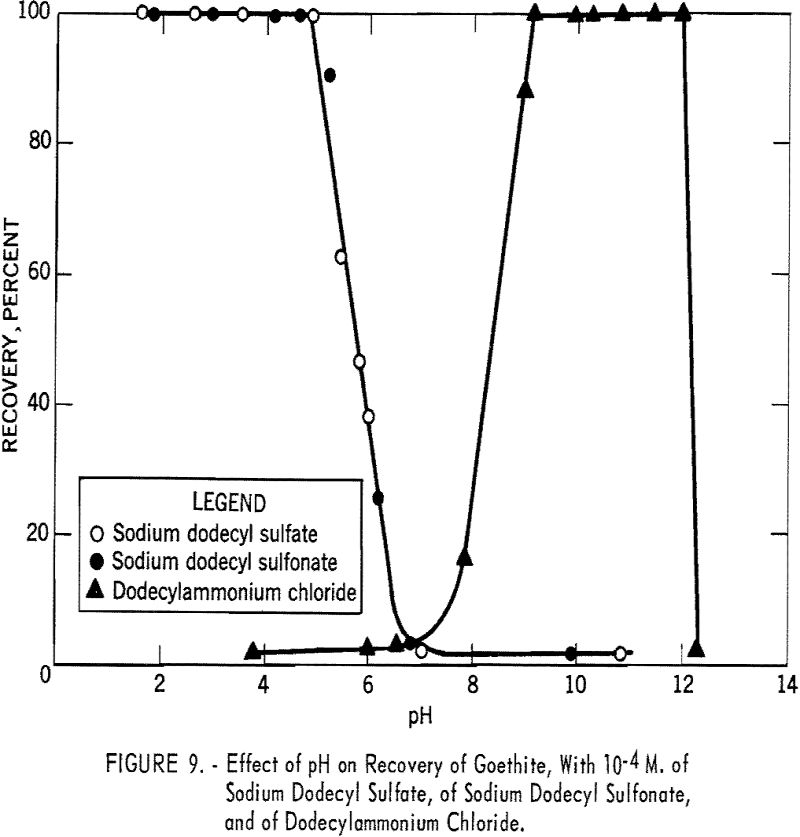
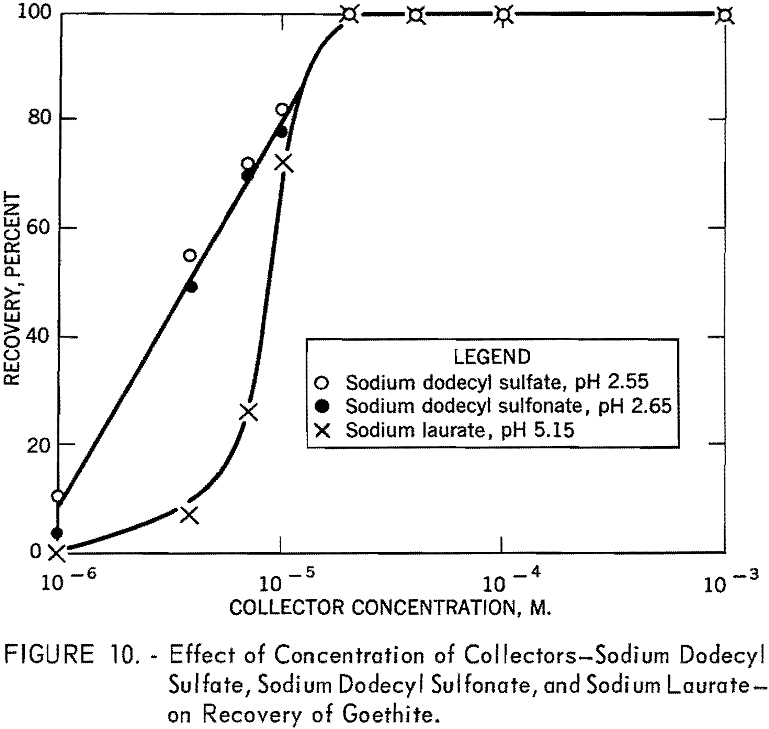
In figure 9 the percent recovery of goethite is plotted against pH using 10 -4 M. of sodium dodecyl sulfate, of sodium dodecyl sulfonate, and of dodecyl-ammonium chloride. As shown, the anionic collectors are effective in acid solution, whereas the cationic collector is effective in alkaline solution. The recovery curve for dodecylammonium chloride crosses the sodium dodecyl sulfate curve at about pH 6.7, the isoelectric point of goethite. The similarity in the action of sodium dodecyl sulfate and of sodium dodecyl sulfonate toward goethite surfaces is further illustrated in figure 10, which shows the flotation recovery as a function of the concentration of these collectors at pH 2.55 and 2.65, respectively. The percent recovery with sodium laurate at a constant pH of 5.15 is also included in the figure.
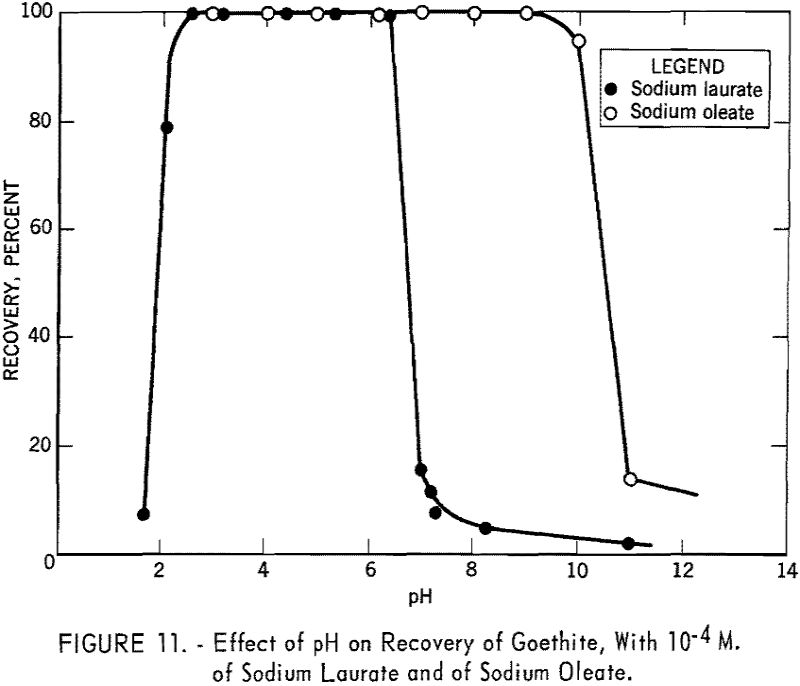
Figure 11 shows the recovery of goethite versus pH curve for 10 -4 M. of sodium laurate. It is evident that this anionic collector is effective only in the acid region. The recovery curve under otherwise identical conditions with 10 -4 M. sodium oleate as obtained by Choi in the laboratories of the School of Mines and Metallurgy, University of Minnesota, is also included in figure 11. A broader pH range of flotation for this 18 carbon-chain collector was obtained as would be expected from the application of Traube’s rule to solid-solution interfaces.
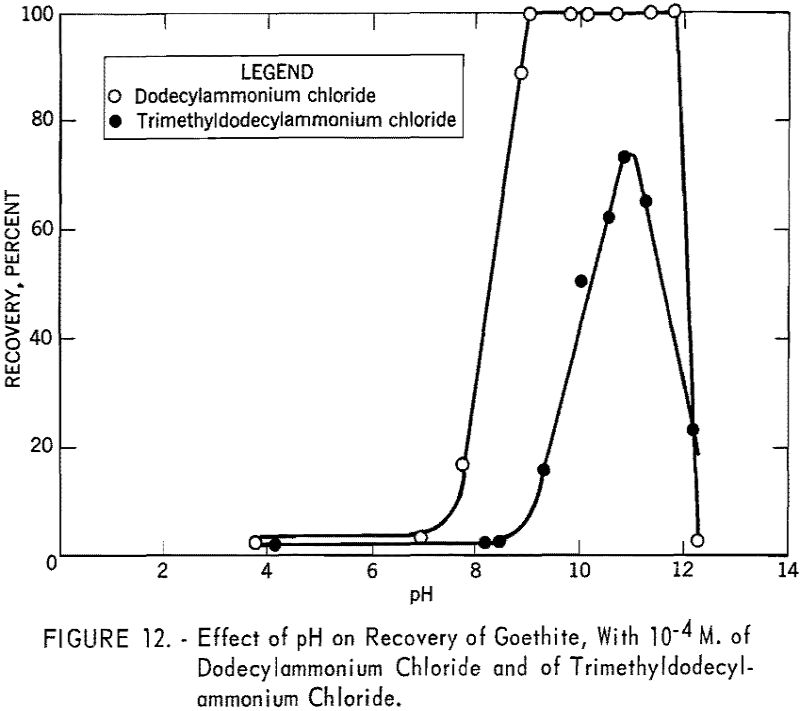
Figure 12 shows the recovery versus pH curves for goethite using 10 -4 M. of dodecylammonium chloride and of trimethyldodecylammonium chloride. With dodecylammonium chloride the recovery drops quite abruptly beyond pH 12, which may be attributed to the depletion of dodecylammonium ion through hydrolysis and percipitation.
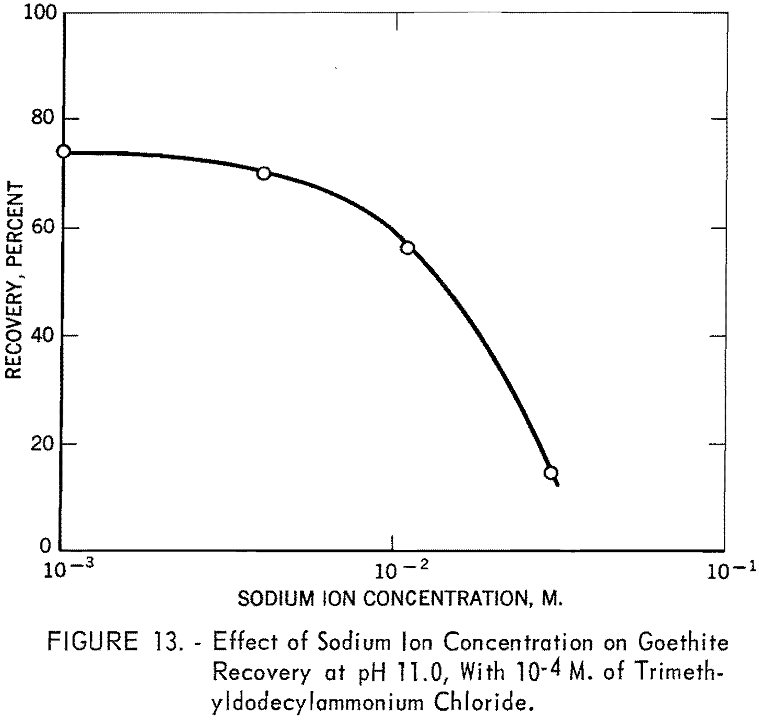
Since trimethyldodecylammonium chloride is known to be more or less completely dissociated over the entire pH range, rapid decrease in recovery with this collector beyond pH 11 was not expected. To investigate the interaction of this collector ion with sodium ion in flotation, a series of tests was made at pH 11.0 using 10 -4 M. trimethyldodecylammonium chloride in the presence of various quantities of sodium chloride. The solution also included sodium ions added as sodium hydroxide for pH regulation. The results are shown in figure 13, from which it is evident that sodium ion has a marked depressing effect on goethite under the specified conditions and is responsible for the decrease in recovery shown by the trimethyldodecylammonium chloride curve in figure 12.
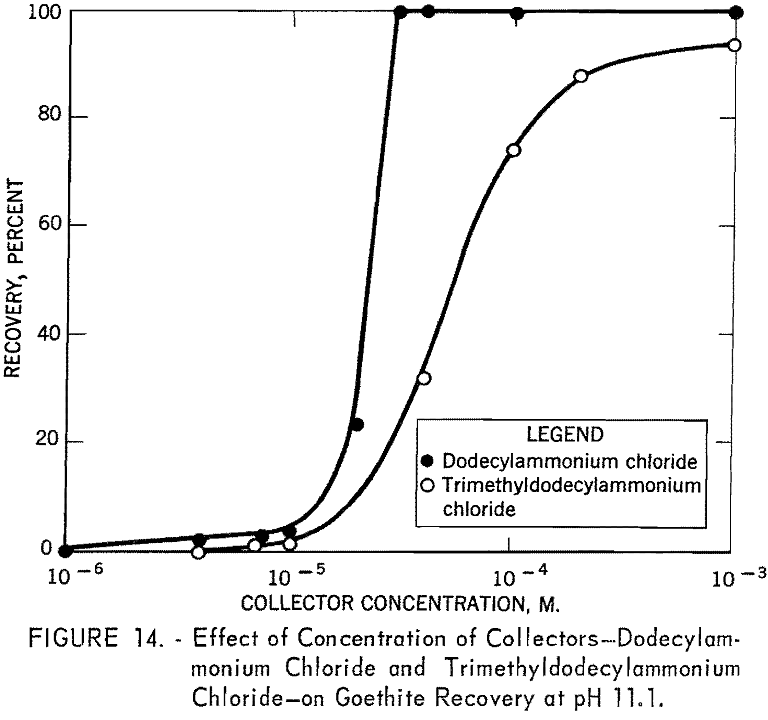
Concentration versus recovery curves for dodecylammonium chloride and tri-methyldodecylammonium chloride at pH 11.1 are presented in figure 14. Lower recoveries with trimethyldodecylammonium chloride indicate that trimethyldodecylammonion ion interacts with goethite surfaces less strongly than dodecylammonium ion interacts.
Hallimond Tube Tests on Quartz
Since one of the major constituents of the gangue in iron-bearing materials is quartz, information on the flotation properties of this mineral under similar conditions to those of goethite was desirable. The electrokinetic behavior of quartz has been investigated by Gaudin and Fuerstenau, who reported the 3 isoelectric point to occur at pH 3.7.
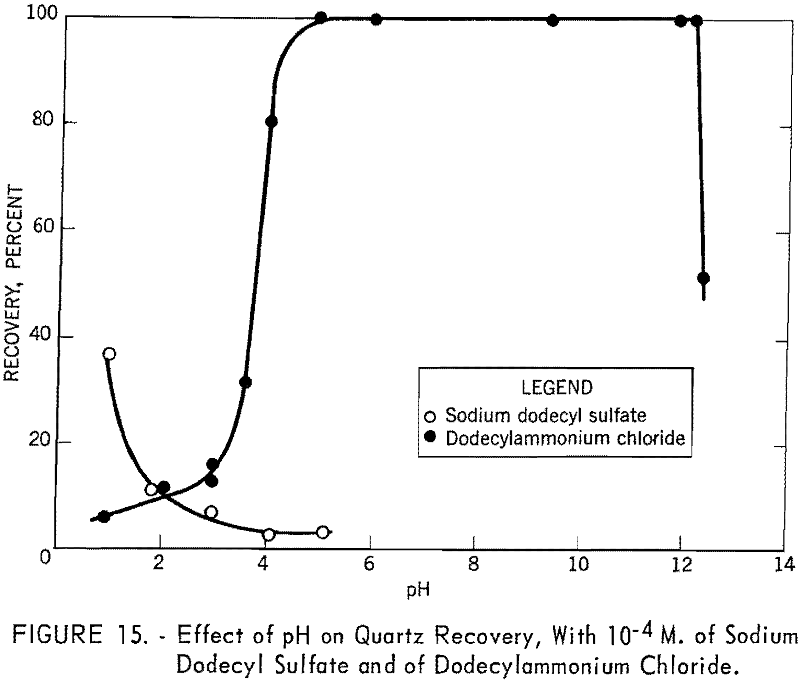
The effects of pH on the recovery of quartz with 10 -4 M. of dodecylammonium chloride and of sodium dodecyl sulfate are shown in figure 15. It is evident from the figure that dodeeylammonium chloride is an effective collector for quartz over a wide range of pH, whereas sodium dodecyl sulfate is a very poor collector at any pH. The figure also shows that the two curves intersect at about pH 2. This may be correlated with the recent investigation of Li, who reported the isoelectric point of quartz to lie between pH 1 and 2.
Batch Flotation Tests on a Mixture of Quartz and Goethite
Inspection of the results of the Hallimond tube tests on goethite and on quartz (figs. 9 and 15) indicates that a mixture of these two minerals may be separated by floating quartz with dodecylammonium chloride in the pH region of 4 to 8 or by floating goethite with sodium dodecyl sulfate below pH 5. To test the applicability of the results obtained with the Hallimond tube, a batch flotation cell was constructed after that devised by Chang, Cooke, and Huch. The cell consisted of a 150-ml. Buechner fritted glass funnel, a stainless steel baffle, and a glass T-impeller. A 20-gram sample containing 60 percent Montana quartz and 40 percent Cuyuna goethite, both 100/150-mesh in size range, was used for each test with approximately 135 ml. of collector solution. Purified nitrogen was used for aeration.
For simplicity in plotting, the results of the batch flotation tests have been expressed in terms of selectivity index, SI, defined as follows :
SI = √M/m x n/N……………………………………………………(2)
where M is percent Fe in concentrate, m is percent Fe in tailing, N is percent silica in concentrate, and n is percent silica in tailing. Selectivity index gives a relative measure of the effectiveness of separation for a two-mineral system. If there is no separation, the selectivity index is unity; whereas, if the separation is perfect, the selectivity index is infinite.
Figure 16 shows the plot of the selectivity indexes of the batch flotation tests as a function of pH in 10 -4 M. solutions of sodium dodecyl sulfate and of dodecylammonium chloride. With the former, the selectivity index reaches a maximum at pH 2, where the concentrate analyzed 49.61 percent Fe and 18.24 percent acid insoluble, with an iron recovery of 97.6 percent. With dodecylammonium chloride as collector, the best separation was obtained at pH 6.4, where the concentrate contained 57.99 percent Fe and 4.68 percent acid insoluble, with an iron recovery of 92.3 percent. Both results agree with the Hallimond tube tests. With the cationic collector and in the alkaline range, both quartz and goethite were expected to float, giving a selectivity index of close to unity, as indicated by the dotted line. Beyond pH 8.5, the experimental results, however, deviated considerably from those expected. The selectivity exhibited in this pH range may be attributed to the nature of the froth; bubbles were smaller and not very persistent, and the goethite particles remaining in the cell tended to film but showered back into the pulp as soon as they reached the surface. To stabilize the froth, Barrett No. 4 was added to the pulp at pH 9.2. The entire sample then floated, as would be expected.
Figure 17 shows the results of batch flotation tests made on the mixture of quartz and goethite in the presence of different quantities of goethite
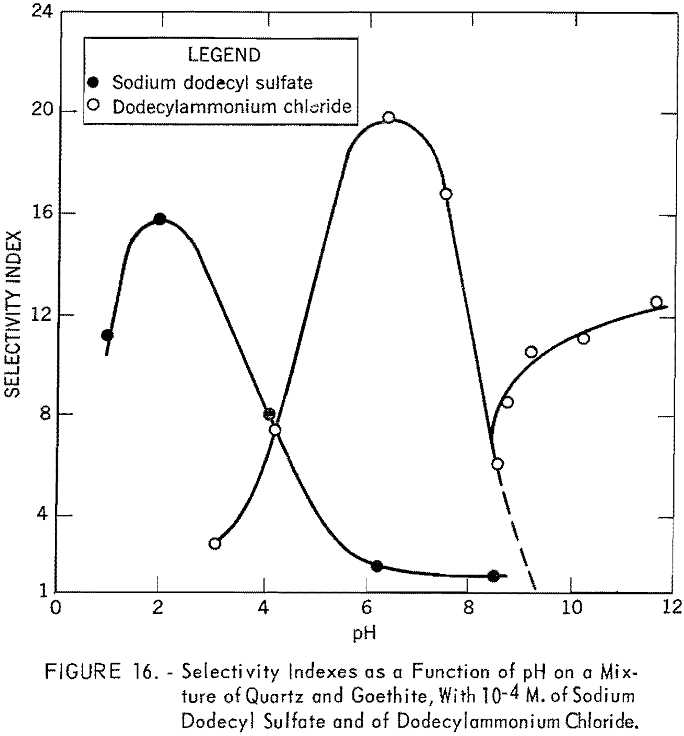
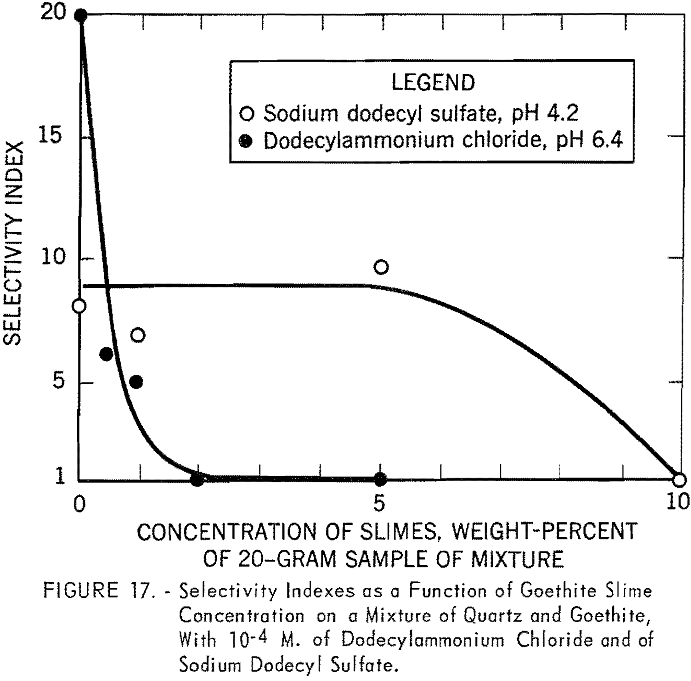
slimes. Concentrations of 10 -4 M. of dodecylammonium chloride and sodium dodecyl sulfate were used in these tests. The pH of the solutions was fixed at 6.4 for the cationic collector and at 4.2 for the anionic collector. In cationic flotation of quartz, the addition of a very small quantity of goethite slimes affects the separation. Flotation of the quartz is almost completely inhibited in the presence of only 0.5 percent slime. However, more than 5 percent goethite slime can be tolerated in the anionic flotation of coarse goethite particles.
Discussion
When mineral particles are dispersed in water or in aqueous solutions and an ionic equilibrium is reached, it is observed that the particles generally migrate under the influence of an electric field. The migration of the mineral particles implies the existence of an electrical charge at the mineral solution interface. Those ions, which establish equilibrium at the interface and which determine the potential drop be¬tween the solid and the liquid phases, are termed “potential determing ions” and are responsible for the electrical charge observed on the solid surface.
For oxide systems, it is postulated that the oxygen ions in the surface undergo the following reaction;
![]()
It follows that hydroxyl ions (and, accordingly, hydrogen ions) are involved in the electrolytic reaction at the surface and are expected to be the potential determining ions.
Streaming potential measurements on goethite as a function of pH and in the presence of different concentrations of sodium chloride (fig. 2) substantiate this postulate. An increase in pH drives the reaction to the left and the concentration of oxygen ions at the surface should increase, thus making the surface more negative, A decrease in the pH should cause the reaction to proceed to the right and the surface oxygen ion concentration would decrease. At a certain pH, conditions should be such that the surface is electrically uncharged. This condition of electrical neutrality is referred to as the isoelectric point. In the present investigation the isoelectric point of goethite was found to lie at pH 6.7.
From fundamental studies of the electrical double layer at a mercury-solution interface, Frumkin concluded that the adsorption of organic cations occurs mainly on a negatively charged surface, of anions on a positively charged surface, and of neutral molecules near the point of zero charge. Similar conclusions were reached in recent investigations on silver iodide and silver sulfide systems by the electrochemical titration technique and on silica and corundum systems by the streaming potential technique. The respective investigators have shown that the surface electrical conditions of solids can be related to the adsorption of organic electrolytes and hence to the floatability of the solids.
The flotation behavior of goethite as investigated by the vacuum flotation tests, contact angle measurements, and Hallimond tube tests and reported in this paper demonstrates that a cationic collector was most effective on a negatively charged surface and an anionic collector on a positively charged surface. The same correlation is obtained with the floatability of quartz when the published data on the electrokinetic behavior and the present flotation results are compared. From the above observation it is apparent that the electrical nature of a mineral surface and of a collector ion controls their interaction and, hence, the flotation behavior.
Modulation of the collector action on mineral surfaces through pH regulation can be interpreted in terms of competition between collector ion and hydrogen or hydroxyl ion. In the following analysis the adsorption of dodecylammonium ion is assumed to involve a stoichiometric ion exchange with the surface hydrogen ion at constant exchange capacity according to:
![]()
Further, the activities of the reacting ionic species are assumed to be represented by the ordinary concentration units, then the equilibrium constant (K) for the above reaction will be

where ΓRNH3+ and ΓH+ are the adsorption densities of the amine and hydrogen ions, and H+ and RNH3+ are the concentrations of the respective ionic species. Let Γo be the total exchange capacity, and assuming that incipient flotation occurs at constant surface coverage ΓRNH3i+, then equation (5) may be rewritten to
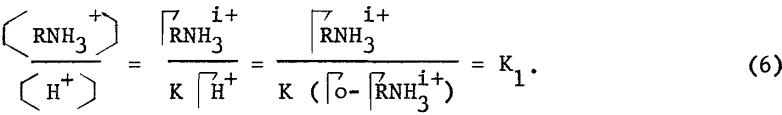
The validity of equation (6) and, hence, the mechanism of stoichiometric ion exchange can be tested by constructing a critical pH curve and determining the slope of the acid limit, which, theoretically, should be unity. From the vacuum flotation results presented in figure 3, the slope of the acid limit of goethite using dodecylammonium chloride is determined to be unity, which agrees with equation (6).
Similarly, for anionic collectors the following relationships may be derived:

Figures 3 and 4 show that the slopes of the alkaline limits are approximately 0.8. These results appear to support the hypothesis of ionic exchange mechanism at the goethite-solution interface.
Contact angle measurements on goethite in the presence of anionic collectors correlate well with mineral floatability as indicated by the vacuum flotation and Hallimond tube tests. With dodecylammonium chloride, however, the contact angle curve rises steeply from pH 7 to a peak of 76° at pH 9.5 and then decreases rapidly to a tendency for the bubble to stick at pH 11.7 (fig. 5). The recovery curve of the Hallimond tube tests under the same conditions, however, shows excellent flotation to pH 12 (fig. 12). This rapid decrease in contact angle beyond pH 9.5 appears at first not to substantiate the Hallimond tube results. Under static conditions, however, it can be shown from the force balance that a contact angle of only a fraction of 1° is required to float a 150-micron (about 100-mesh) goethite particle. In a Hallimond tube, the stirrer action may require a slightly greater angle than static flotation conditions, and the flotation of a 150-micron goethite particle should certainly be effected with a contact angle of not more than a few degrees. Since in contact angle measurement, a tendency to stick would indicate an angle of less than 10°, good flotation with a Hallimond tube could be feasible between pH values of 9.5 and 12.
From the Hallimond tube tests with the anionic collectors, the recovery versus pH and the recovery versus concentration curves for goethite show a similar trend (figs. 9, 10, and 11), whereas, with the cationic collectors, dodecylammonium chloride is obviously a more effective collector than trimethyldodecylammonium chloride (figs. 12 and 14). The sizes of polar groups of the organic electrolytes used in this investigation are as follows:
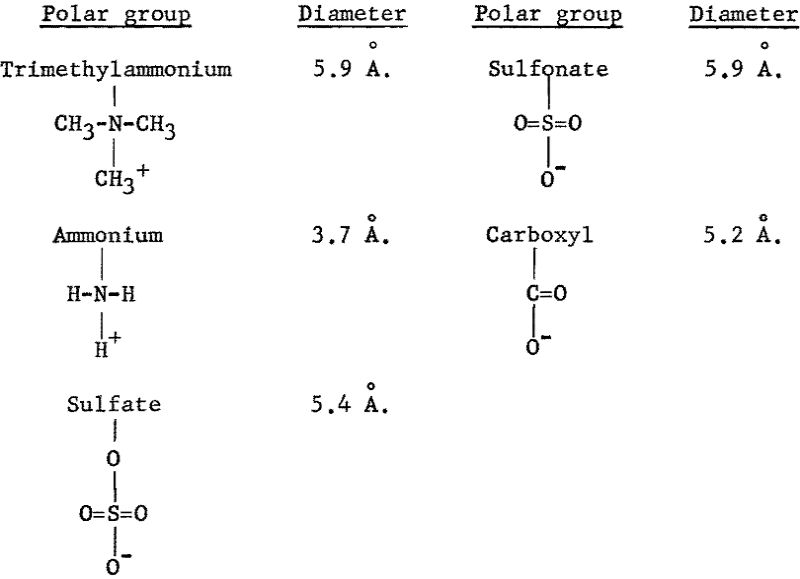
The diameters of the three anionic groups are similar and suggest that the expected interaction between any of these organic anions and the mineral surface would be similar. The considerable difference in the size of the two cationic groups, however, suggests that the interaction of dodecylammonium ions with the mineral surface is stronger; hence, there will be greater adsorption of dodecylammonium ion than trimethyldodecylammonium ion at the same bulk concentration.
Batch flotation tests on an artificial mixture of goethite and quartz with dodecylammonium chloride have shown that the range of best selectivity is from pH 5.5 to 7.5. This substantiates the results expected from the Hallimond tube tests carried out on goethite and on quartz individually. The present observation also agrees with results obtained by Chang, Cooke, and Huch, who floated a Mesabi wash-ore tailing using dodecylammonium acetate as collector and starch as depressant. They reported the optimum flotation range to be from pH 6 to 8.
With sodium dodecyl sulfate as collector the optimum selectivity was observed to be in the range from pH 1 to 3.5, which agrees with the practical experience reported for petroleum sulfonates.
In cationic flotation with 10 -4 M, dodecylammonium chloride, a small quantity of goethite slimes (0.5 percent or the equivalent of 100 mg. per 140 ml. of collector solution) was shown to depress the flotation of quartz from a mixture of quartz and goethite. The flotation tests were made at pH 6.4. At this pH the surfaces of the goethite and the goethite slimes would be slightly positive, whereas those of the quartz are negative. Under such conditions, very few collector ions would be abstracted by goethite and goethite slimes. It was found colorimetrically that at 100 mg. of the same goethite slime per 100 ml. of 10 -4 M. dodecylammonium chloride there was no appreciable abstraction of amine. It can therefore be concluded that the decrease in floatability must be attributed to the slime coating on the quartz surface.
When sodium dodecyl sulfate was used to float goethite from the mixture, the addition of up to 5 percent of goethite slimes had little effect on the flotation of the mineral. At pH 4.2 the goethite and goethite slimes have a positive surface charge. The depletion of sodium dodecyl sulfate by the adsorption on positively charged goethite slimes at this pH may account for the decrease in the floatability of goethite and, hence, a decrease in the selectivity index. The last observation does not conform with industrial practice, where desliming is an essential step for efficient separation with petroleum sulfonates. Further study on the mineralogical composition and on the size distribution of ore slimes may throw some light on this problem.
