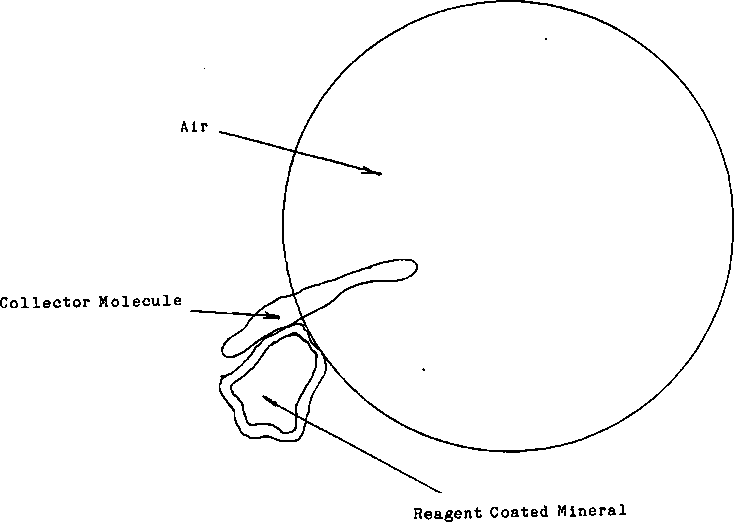
 This branch of chemicals will prepare the mineral surface so other reagents will be able to collect the metal. For Conditioners and Modifiers, it may be simply cleaning the surface of the particles, as in the case of removing oxidization, or it may be a chemical that these are the chemicals that bind the mineral to the air bubble to be lifted away from the waste. Activators will attach itself to the surface of the mineral to allowing other reagents to react to it.
This branch of chemicals will prepare the mineral surface so other reagents will be able to collect the metal. For Conditioners and Modifiers, it may be simply cleaning the surface of the particles, as in the case of removing oxidization, or it may be a chemical that these are the chemicals that bind the mineral to the air bubble to be lifted away from the waste. Activators will attach itself to the surface of the mineral to allowing other reagents to react to it.
Depressants
This is a group of chemicals that react with the minerals to prevent them from floating with the collector.
These four are the main classifications of mining reagents that we will deal with. There are others but they either are special use reagents or are used in other processes. These four types of reagents are basic for use in a flotation circuit.
Modifiers
The last group of reagents that we are going to discuss are the modifiers, these are reagents that are used to change the characteristics of a mineral so that the interaction between mineral and collector may take place. These would be used only in an application where the attraction between the mineral and the collector doesn’t exist. A modifier provides the interface that both collector and mineral may relate to.
That is a brief explanation as to how those four main groups of reagents will relate to one another, as a brief review, let’s go over the interaction quickly. First there are DEPRESSANTS, they are added to prevent the unwanted minerals from floating. Next comes the COLLECTORS this reagent is used to gather the minerals. This reagent will attach itself to an air bubble while it is also attached to the mineral, as the air bubble rises to the surface of the slurry it will lift the mineral away from the waste. Another important reagent group are the FROTHERS. They strengthen the surface of the air bubbles to prevent breaking. Lastly, the MODIFIERS. They provide a common surface that both collector and mineral will be able to attach themselves to.
As a demonstration between each of the reagents and the mineral let’s recover a hypothetical mineral. For this exercise let’s pretend that the ore that we are recovering is chalcopyrite. This is a common mineral consisting of approximately 35% copper, approximately 32% Iron, approximately 32% Sulphur, and % Miscellaneous. It is expressed chemically as CuFeS2. It may be found in association with other copper bearing minerals like Bornite and Chalcocite or non-copper bearing minerals like Galena, Zinc, Silver and Gold. To start with, let’s keep this simple. The ore body that we will be concentrating is composed of iron pyrite and chalcopyrite with trace minerals of gold. A TRACE MINERAL is a mineral that isn’t in a large enough quantity in the ore body to warrant recovery as a principal mineral but may have an impact on the total value of the ore body.
The goal of flotation is the SEPARATION and RETRIEVAL of the valuable metals from the unwanted minerals and waste rock. Once we have identified the mineral to be collected, in this case chalcopyrite, we will find a collector that will react with it. While the decision is being made in regards of which collector is to be used, it has to be kept in mind that the minerals that are in association with the chalcopyrite will be effected as well. In this case Iron pyrite and gold.
As Iron pyrite (FeS2) and chalcopyrite (CuFeS2) are very similar the collector will be attracted to both of them. Gold on the other hand is not of the same mineral composition meaning of course that the collector will not attach itself as easily to gold. Iron pyrite unfortunately does not have a market value, therefore is an unwanted mineral. To prevent this mineral from being floated requires a collector that is selective towards the copper as well as an additional reagent to depress the Iron pyrite.
The first question is. How will the COLLECTOR and DEPRESSANT react to the ore? Each particle will be either solid rock, solid mineral or a have a varied combination of both the wanted and unwanted material. The first reagent to be added to the ore is the depressant. Then the collector. The effectiveness of the collector will depend upon the degree of mineral liberation, the size of the particles, and the amount of reagents added to the ore.
We stated that the collector is attracted to the mineral composition of the ore. What these reagents do is add the particular mineral characteristic that the collector is attracted to the surface of the mineral to modify its behavior to the collector. An example of this is the gold in our mythical ore. Normally gold is not attracted to the same reagents that chalcopyrite is, but by adding a modifier it will become sensitive to same reagents as the chalcopyrite. If we were using a modifier, too little of the modifying reagent would result in a loss of the mineral. This would be reflected in froth color and bubble size. Both are dependent upon the mineral that is being collected. If however there is too much of the modifying agent being used then the froth MAY or MAY NOT reflect this in color as well. The reagent being used and the mineral being collected will dictate the characteristics of the indication.
