Table of Contents
The Bureau of Mines undertook a research program to insure an adequate supply of iron raw materials for future iron and steel making needs. A sample was obtained of oxidized taconite from the western Mesabi Range in Minnesota, and the metallurgical response of the taconite to beneficiation by a process that included selective flocculation-desliming-cationic flotation was evaluated. The sample contained 35.3 pct iron and represented approximately 2 billion tons of crude material. Selective flocculation and flotation were evaluated in a pilot plant with a feed rate of 900 lb/hr. Concentrates averaging 61. 7 pct iron and 5.1 pct silica were obtained, with an accompanying iron recovery of 67.2 pct. About 83 pct of the process water requirements were reclaimed from the slime and froth tailing and then recycled.
The United States imported approximately 29 pct of its ore requirements for iron and steel making in 1978 and will require increased tonnages of imports as the steel industry expands to meet future needs. Most of the domestic raw materials used in 1978 came from pelletized magnetite concentrates. However, as the demand for iron-bearing raw materials increases, western Mesabi Range oxidized (nonmagnetic) taconites, which are a major domestic resource, containing an estimated 10 billion tons of material assaying 30 to 40 pct iron, will have to be used. The oxidized taconites cannot be beneficiated by the wet low-intensity magnetic separation method used to upgrade magnetite taconites. Therefore, alternative beneficiation processes for treating the oxidized taconites are being evaluated at the Twin Cities (Minn.) Research Center of the Bureau of Mines to provide the technology to insure an adequate future supply of iron raw materials for the iron and steel industry.
In 1973, the Bureau began a comprehensive program on the beneficiation of western Mesabi Range oxidized taconites. The objectives of the investigation were (1) to increase availability of domestic iron ores through demonstrated amenability of oxidized taconites to beneficiation, (2) to make an economic and metallurgical comparison of the alternative processes; selective flocculation-flotation and reduction roasting-magnetic separation-flotation, and (3) to develop new and improved beneficiation methods for oxidized taconites. The iron potential of the western Mesabi Range oxidized taconites was to be evaluated by taking three bulk samples, containing 350, 600, and 800 tons, respectively, with different mineralogical makeups and representing large blocks of material. The metallurgical responses of these samples to the two alternative processes previously mentioned were evaluated. Both processes have been investigated, but require further development to reduce processing costs and to establish that they are applicable to the range of ore types likely to be encountered. This report summarizes the data obtained from the application of the Bureau-developed selective flocculation and flotation process to the first of the three bulk samples.
Sample Description
A 350-ton sample, which represents about 2 billion tons of oxidized taconite, was obtained from the iron formation located near Nashwauk, Minn., and crushed to a nominal minus 5/8-inch size. Table 1 shows the mineral distribution in the bulk sample. The iron oxides in the sample were primarily hematite and goethite; hematite-to-goethite ratio was 1.1:1. Most of the hematite and magnetite grains were from 30 to 300 µm and averaged about 100 µm. The goethite particles ranged from a few micrometers to 1.5 mm and averaged approximately 40 µm. The hematite pseudomorphically replaced magnetite, thus preserving the original shape of the octahedral magnetite, which usually occurred in clusters with granule shapes and occasionally occurred as single grains. A small amount of hematite replaced siderite and iron silicates; occasionally, goethite replaced bladed silicates. Most of the goethite was present as irregular shapes because it filled cavities where quartz or chert was leached out. Much of the goethite was also coarser than the hematite and magnetite and had no apparent crystal outline or habit. The magnetite occurred as unaltered grains and unoxidized cores in hematite particles. Liberation of the iron oxides from the gangue minerals was between 20 and 50 µm. Table 2 shows the average chemical analysis of the taconite, and an average screen analysis of the crude taconite is given in table 3.

Pilot Plant Research
Methods for upgrading the fine-grained oxidized taconites found in the Michigan and Minnesota iron ranges include the selective flocculation-gangue flotation system using either an anionic or a cationic collector. After extensive research and development on the two flotation systems, the cationic flotation process was determined to be the simplest system to apply commercially. The selective flocculation-cationic flotation system includes (1) stage grinding of the oxidized taconite, with dispersants to liberate the iron minerals from the gangue, (2) selective flocculation of the iron minerals with starch, (3) rejection of a siliceous slime, and (4) finally, cationic flotation of the selectively flocculated material to yield a froth tailing and an iron oxide concentrate.
Flowsheet, Equipment, and Test Conditions
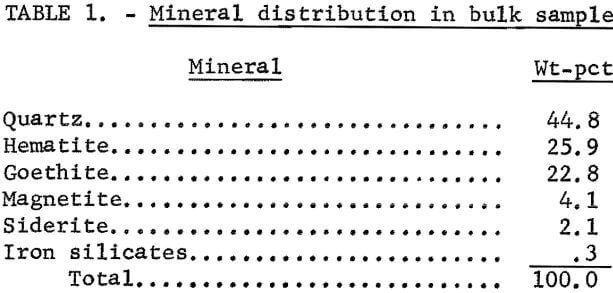
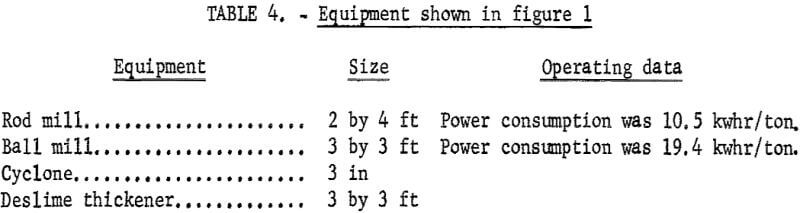
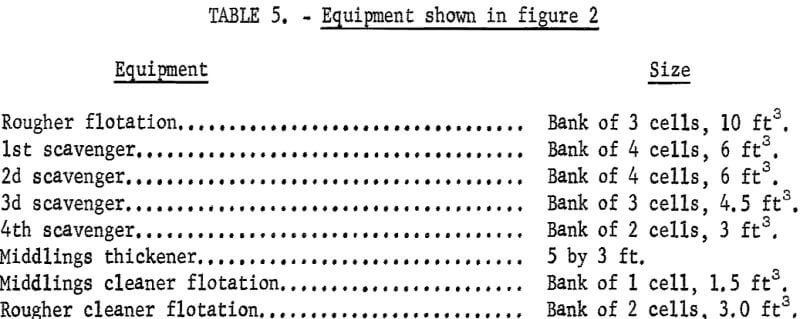
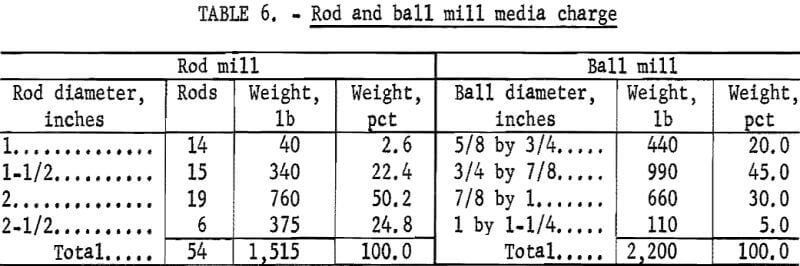
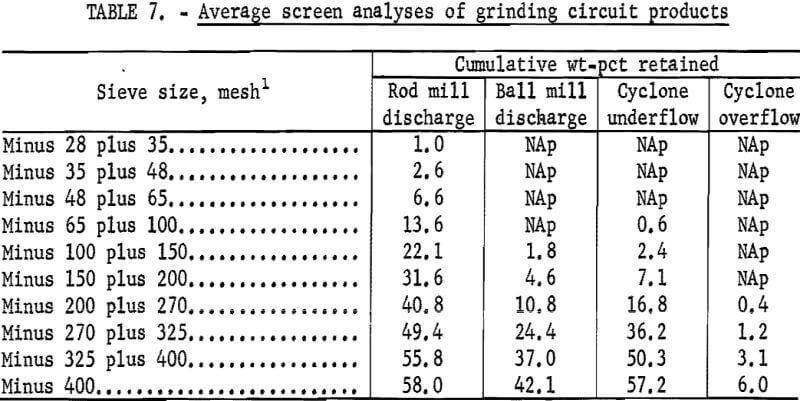
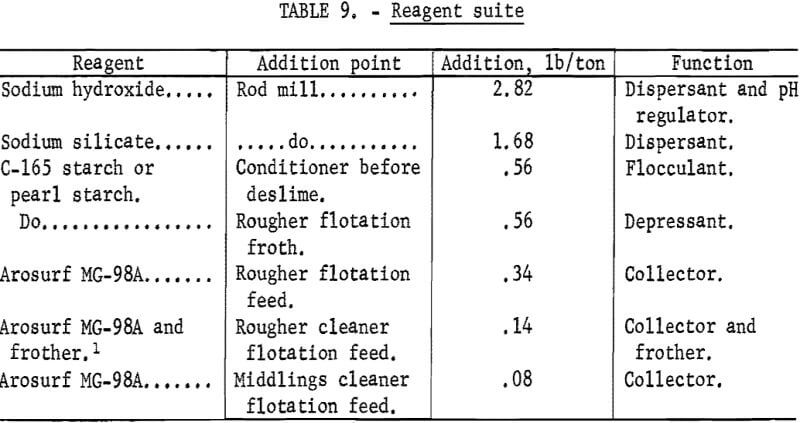
Figures 1 and 2 show the selective flocculation and flotation process and include the metallurgical data obtained during the operation of the pilot plant. Tables 4 and 5 show the equipment used in the pilot plant research. Minus 5/8-inch oxidized taconite at 900 lb/hr was diluted to 70 pct solids with reclaimed water and fed to a rodmill containing a seasoned charge of hot-rolled carbon steel rods (table 6). Sodium hydroxide and sodium silicate were added to the rodmill to disperse the pulp and to regulate the pH at 11. A rod wear rate of 2.1 lb/ton was obtained during grinding to 42 pct minus 400 mesh. The average screen analysis of the rodmill discharge and other products in the grinding circuit are reported in table 7. Rodmill discharge was pumped to an overflow-type ball mill, which ground the taconite to 58 pct minus 400 mesh. The seasoned charge of balls shown in table 6 had a wear rate of 4.4 lb/ton. Ball mill discharge was diluted to 42 pct solids with reclaimed water and then sized in a hydrocyclone. Cyclone underflow, averaging 488 wt-pct of the plant feed, was returned to the ball mill for regrinding. Cyclone overflow, averaging 94 pct minus 400 mesh, was conditioned with starch to
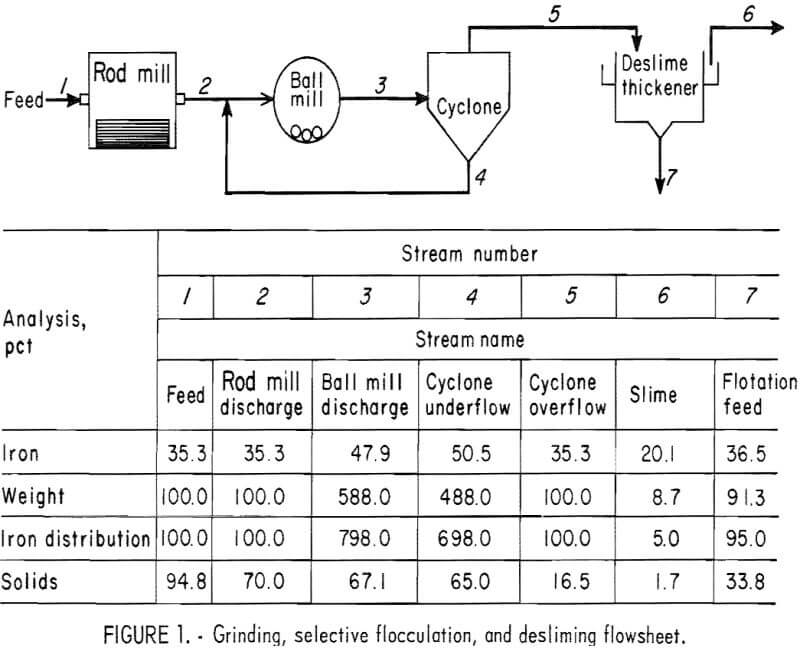
selectively flocculate the iron oxides and then fed into a thickener. A slime product was separated at a rise rate of 1.6 in/min and was routed to the water treatment circuit. The thickener underflow was routed to the rougher flotation cells where Arosurf MG-98A, a cationic collector, was added to the flotation feed box. Froth from the rougher flotation step was scavenged in four scavenger flotation steps. Starch was added to the rougher flotation froth to depress the iron oxides. The four scavenger underflows or middlings were thickened and then cleaned in a single flotation cell. A small amount of MG-98A collector was added to the middlings cleaner flotation step. The middlings thickener, which overflowed at a rate of about 6.7 gpm, was used to dilute the cyclone feed. The rougher concentrate was reagentized with a collector-froth mixture with a collector-to-frother ratio of 100:1 and up-graded in the rougher cleaner flotation cells. The underflows from the rougher and middlings cleaner were combined and became the final concentrate. The cleaner froths were returned to the rougher flotation step.
Metallurgical Results
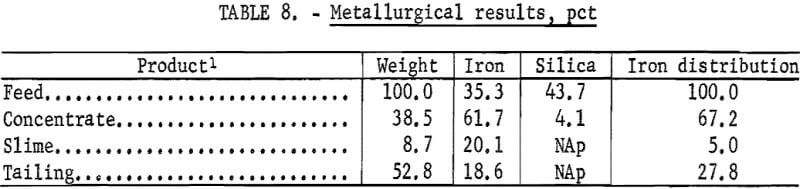
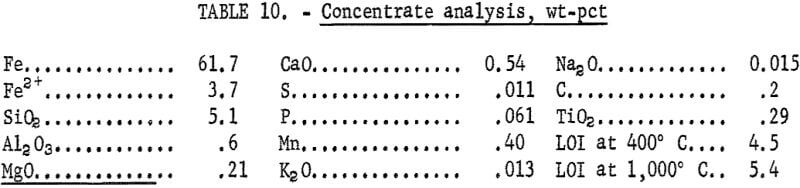
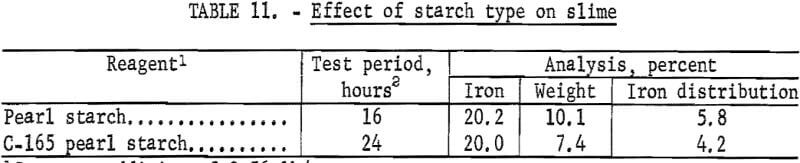
The metallurgical results shown in table 8 are averaged analyses of the concentrate, slime, and tailing obtained from 40 consecutive hours of pilot plant operation. The average reagent suite utilized is given in table 9. Samples of the pilot plant streams were taken when equilibrium was achieved, or about 4 hours into the shift; five sample cuts were taken during a 1-hour period. The concentrate had an average grade of 61.7 pct iron and 5.1 pct silica with recovery of 67.2 pct of the iron in the feed. Table 10 is an average chemical analysis of the concentrates obtained. The slimes analyzed 20.1 pct iron and contained 5.0 pct of the iron in the feed. Table 11 shows the results obtained from using two types of starches in the selective flocculation step. Causticized pearl starch and C-165 pearl starch, which is similar to a jet-cooked pearl starch, gave similar results. The tailing averaged 18.6 pct iron and contained 27.8 pct of the iron in the feed.
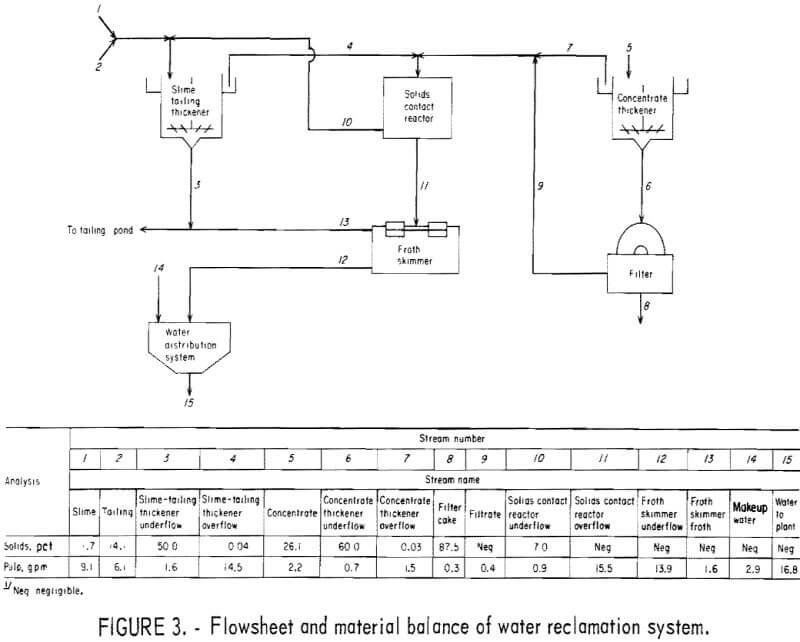
Water Reclamation
Figure 3 shows the water reclamation flowsheet used to treat the pilot plant waste streams, and the water reclamation equipment that was used is shown in table 12. Slime and froth tailing were combined and gave a 7.0 pct solids slurry, which was flocculated with 1.68 pounds of lime/ton and 0.035 pound of a cationic flocculant Superfloc 330 per ton and then fed to a thickener. The thickener overflow was pumped to a solids contact reactor (SCR), which is a rectangular, compartmented water treatment tank that employs the principles of sludge blanket clarification. The SCR underflow at 7.0 pct solids was recycled to the slime-tailing thickener, and the overflow was pumped to a froth skimmer to remove excess froth, which was pumped to the tailing pond. Slime-tailing thickener underflow, which was controlled at 50 pct solids to prevent excess water loss, was pumped to a tailing pond.

Iron oxide concentrate was pumped to a thickener and flocculated with 0.035 pound of Superfloc 330 per ton. The thickener underflow was controlled at 60 pct solids and filtered on a rotary drum filter. Filter cakes with an average moisture of 12.5 pct and Blaine surface area of 2,855 cm³/g were obtained. The filtrate was combined with the concentrate thickener overflow and pumped to the SCR.
The froth skimmer underflow was pumped to a water distribution tank, where makeup water was added to satisfy the requirements of the plant. An average of 83 pct of the total water used, or 13.9 gpm, was recovered and recylcled. The reclaimed water had an average turbidity of 1,360 ppm silica equivalent, pH of 10.8, and calcium ion content of 6.7 ppm.
Estimated Reagent Costs
Table 13 shows the estimated reagent cost for the selective flocculation and flotation beneficiation of the oxidized taconite. The reagent unit costs are based on bulk quantities. Reagent cost for selective flocculation and desliming was 32.7 cents/ton, flotation reagent cost was 40.9 cents/ton, the water reclamation cost was 8.7 cents/ton, and total reagent cost was 82.3 cents/ ton. At a 38.5 pct weight recovery, the reagent cost per long ton of concentrate would be $2.14.

Summary and Conclusions
The first phase of the Bureau’s research effort to insure a future domestic supply of iron raw materials for the iron and steel industry was the evaluation of a bulk sample representing an estimated 2 billion tons of oxidized taconite and containing about 35.3 pct iron. The oxidized taconite had a 1.1:1 hematite-to-goethite ratio and was beneficiated by selective flocculation and cationic flotation as an alternative method for recovering the iron oxides. Concentrates averaging 61.7 pct iron and 5.1 pct silica, with an accompanying iron recovery of 67.2 pct, were made. At a 38.5-pct weight recovery, the reagent cost per long ton of concentrate was $2.14. The metallurgical results are similar to those that can be expected in a commercial operation.
