The object of the Fire Assay Fusion is to concentrate the precious metals in a button of lead, while the whole of the remainder of the ore forms a fusible slag with suitable fluxes, in which lead sinks. The fusion is made in clay (or rarely iron) crucibles in a wind furnace, similar to, but usually smaller than, that used for melting bullion. Coke is generally used for fuel. Gas and petroleum furnaces are also in wide use.
In Colorado it is usual to make fusions in a muffle furnace, similar to that described below under cupellation. The temperature required is about the same as that used in scorification. The advantages claimed are greater cleanliness and neatness and more uniformity in the conditions, the temperature of a muffle being more easily kept constant and uniform than that of an ordinary fusion furnace. Six or eight Fire Assay Fusions can be made at one time in a large muffle.
In weighing the materials for a crucible charge the use of a set of assay-ton weights saves much labour in calculation. The assay-ton is a weight which contains as many milligrammes as a ton contains ounces. Thus an English or long ton of 2,240 lbs. (used in England and Australia) contains 32,666 Troy ounces, so that the corresponding assay-ton must weigh 32,666 mg. or 32.666 grams. If the weight of the resulting bead of gold (or silver) from an assay-ton of ore is 1.5 milligrammes, then the ore contains 1.5 ozs. of gold per statute ton. If the value per short ton of 2,000 lbs. (used in North America and Africa) is required, the weight of the assay-ton is 29.166 grammes, since there are 29,166 Troy ounces in 2,000 lbs. avoirdupois. If grain weights are preferred, the weight of the assay-ton may conveniently be taken as 326.66 grains (or 291.66 grains for the short ton) ; the weight of the resulting bead of gold in hundredths of a grain then gives the value of the ore in ounces per ton.
Sets of weights ranging from 1/5 A.T. (assay-ton) to 4 A.T. can be bought, or they can be made up from an ordinary box of decimal weights. In the following pages this system is used, one A.T. being equal to 32.666 grammes.
If grammes or grains are used as units, tables are necessary to convert the results obtained by the balance into ounces per ton. The amount of the unit used in weighing should not be lost sight of in the course of conversion. Thus if 50 grammes of ore are taken, and the resulting gold weighs 1.75 milligrammes, the unit of weight being 0.05 milligramme, the balance error corresponds to nearly 8 grains per ton, and the report should be 1 oz. 3 dwts. per ton, not 1 oz. 2 dwts. 20.8 grains as would probably be given in a table.
The weight of ore taken for assay varies according to circumstances. One A.T. is a suitable quantity if the value in gold is from 0.5 oz. to 10 ozs. or more per ton, and the balance for the final weighings is sensitive to 0.02 milligrams or less. With poor residues, 2, 4, or even 12 A.T. may be taken, and with very rich ores ½ A.T. may suffice. If more than 3 A.T. of ore are taken, the charge is divided between two or more pots, and the resulting lead buttons scorified down and cupelled together.
https://www.youtube.com/watch?v=sJJneyTq7Mw
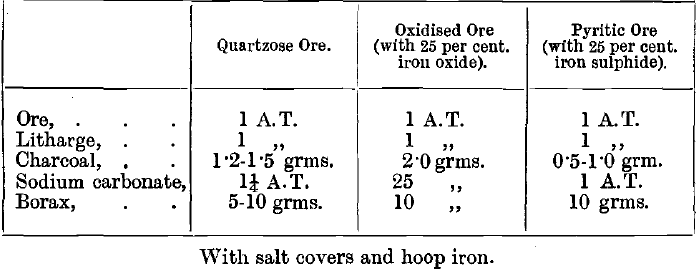
The charge is made up of litharge, charcoal, and suitable fluxes, together with metallic iron. Litharge or red lead is added in the proportion usually of one and a-half or two parts to two of ore. The amount of lead reduced should be from 25 to 30 grammes per A.T. of ore.
The amount of the charcoal added varies with the reducing power (percentage of ash) of the particular sample which is employed, as well as with the degree of oxidation of the ore. If too little charcoal is used, the amount of reduced lead will be too small, and if too much charcoal is added, the charge remains pasty and emits bubbles of combustible gas (CO). Generally from 1 to 1½ grammes per A.T. of ore are enough, but in some highly basic oxidised ores or roasted pyrites as much as 3 grams of charcoal powder are required for 1 A.T. of ore, as the oxides must always be completely reduced to the lowest state of oxidation (e.g., FeO) compatible with keeping them in the slag. This point is attained when nearly all the litharge (about 90 per cent.) is reduced to lead. Otherwise some of the gold is oxidised and retained in the slag. If ores contain much sulphur no charcoal is used, and nitre may even be added to burn off the excess of sulphur, but in that case the pot is liable to boil over. With very large quantities of sulphides it is better to increase the quantity of soda, or, if the formation of a matte cannot be prevented in this way, to treat the matte separately, or to roast the ore before fusion. Flour and argol (acid potassium tartrate) may be used as reducing agents instead of charcoal. Their reducing power is tested beforehand.
Carbonate of soda is used to flux silica and to take up sulphur, while borax is valuable in basic ores to prevent corrosion of the crucible, and render the slag more liquid. The relative amounts required are judged from the appearance of the ore in the first place, and afterwards modified according to the success of the Fire Assay Fusion. From ¼ to ½ A.T. of sodium carbonate, and from ¼ to ½ A.T. of borax to 1 A.T. of ore are the amounts usually required. Even when the ore is entirely composed of silica, some borax is added. The most convenient form is borax glass. Bicarbonate of soda is less convenient than the normal carbonate. If it is used the water and excess carbonic acid must be driven off by slow and cautious heating.
Silica is used only for ores full of lime, baryta, compounds of the base metals or generally whenever the ore does not contain much quartz. It aids fusion in these cases, and protects the crucible from corrosion. From ¼ to ½ A.T. of silica to 1 A.T. of ore is generally enough. About two parts of glass may be used instead of one part of silica. Fluorspar is added to the charge when the ore contains sulphates of barium or calcium, and in the fusion of cupels. Like borax, it increases the fluidity of almost any charge, but it attacks the crucible, and care must be taken to avoid deficiency of silica when it is used. In general it may be noted that for basic impurities an acid flux is used and for an acid gangue a basic flux. A cover of common salt is generally used to protect the charge from the variable oxidising or reducing action of the atmosphere in the furnace. Iron is added to all charges in the shape of large nails or hoop-iron, or even scrap. Sulphur, arsenic are thus kept out of the lead button. The charge for any given ore is made up by the assayer according to his judgment and experience. Much may be learnt as to the nature of the ore by the examination with a lens of unbroken lumps and, after panning, of pulverised ore. The following are some typical charges: