Table of Contents
- Fluxing and Fusion of the Sample
- Basic Fluxes
- Sodium carbonate (Na2CO3) or Soda ash
- Sodium bicarbonate (NaHCO3) baking soda
- Litharge
- Hematite (iron oxide – Fe2O3)
- Lime (CaO)
- Acid Fluxes
- Borax Glass (sodium tetraborate – Na2B4O7)
- Silica (SiO2)
- Neutral Fluxes and Neutral Sample
- Fluorspar (calcium fluoride – CaF2)
- Salt (sodium chloride – NaCl)
- Cryolite (AlNa3F6)
- Reducing Fluxing Reagents
- Flour
- Tartar (potassium bitartrate – KHC4H4O6)
- Charcoal
- Basic Fluxes
- Oxidizing Fluxing Reagents
- Desulfurizing Reagents
- The Fluxing and Fusion Process
- Separating the Slag from the Button
- Cupellation
- Parting
- Fire Assaying Platinum group Elements (PGE’s)
- Glossary of Fire Assay Terms
Fire assaying has been practiced since ancient times and has proven to be very reliable in the determination of precious metals. References to fire assaying are found in the old testament of the Bible and artifacts in past civilizations’ ruins. Assaying began to appear in French and English literature in the 12th century and from there spread to the rest of Europe shortly after. The substances that can be fire assayed can be ores, metallic solutions, and any material that is thought to be containing gold and silver. It uses scientific methodology and it is a branch of analytical and inorganic chemistry.

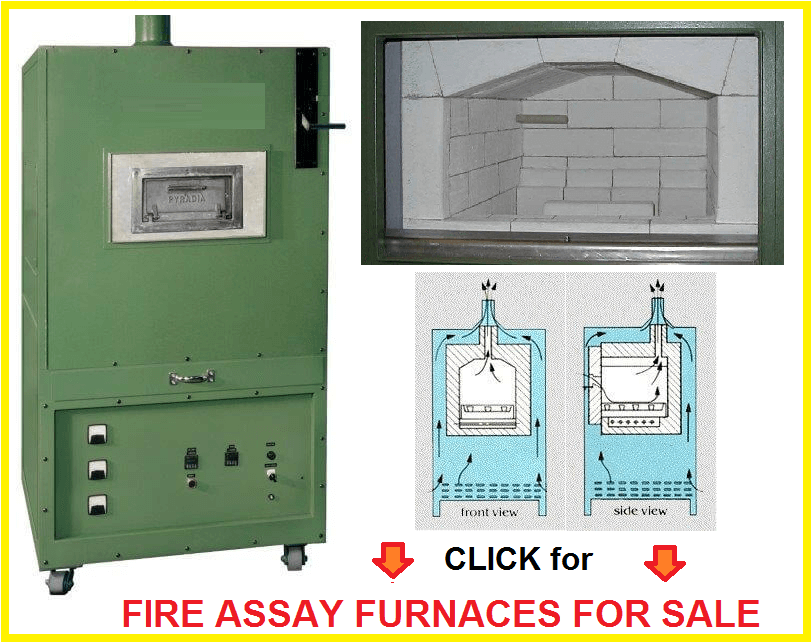
Fire assay procedures use a combination of intense heat (1900 °F) that is produced by a furnace, dry reagents called fluxes, and bone ash containers called crucibles and cupels. One uses this combination to fuse the fluxing agents and to isolate the precious metals. The ability to fire assay a substance relies on a number of facts. First of all, the samples that contain gold and silver should be solubility in the molten metallic lead, and that gold and silver are insolubility in slag. Fire assay also relies on the difference in specific gravity between the two liquids, the molten lead, and the slag. This difference in specific gravity separates the precious metal–bearing alloy from the slag. The precious metals can be isolated by a carefully controlled oxidizing fusion, in a porous container called a cupel. Lastly, fire assay is possible because of the solubility of silver in nitric acid and gold’s insolubility in nitric acid. One requires a certain proficiency in fire assay in order to produce reproducible results. One should assay known pulp standards and run duplicates of the samples to ensure results are correct.
The results are calculated and have a standard measurement unit. In fire assaying one deals with small amounts of gold in a large sample and therefore one uses the unit, an Assay ton for fire assay. The assay ton is 29.166 grams. The assay-ton system is based on the number of Troy Ounces in a Short Ton. The standard units that are reported are grams/ton or oz/ton. One should have some familiarity of the units and the conversions.
Unit conversion factors table.
1 troy ounce = 31.10 grams
1 troy ounce = 1.0971 ounces avoirdupois (av.)
1 ounce (av) = 28.35 grams
1 pound (av) = 14.58 oz (troy)
1 pound (av) = 453.6 grams
1 pound (troy) = 12 oz (troy)
1 pound (troy) = 373.2 grams
1 pound (troy) = 13.17 oz. Av
1 long ton (2,240 lb. av.) = 32,666 oz. troy
1 short ton (2,000 lb. av.) = 29,166 oz. troy
1 metric ton (2,204.6 lb. av.) = 32,150 oz. troy
1 kg = 32.15 oz. troy
1 kg = 2.68 lb. troy
1 kg = 35.27 oz. av
1 kg = 2.20 lb. av
1 ppm = 1 mg/kg
1 ppb = .001 mg/kg
There are a number of different procedures and methods that are used in the fire assay of precious metals. The precious metals that can be fire assayed are gold, silver, platinum, palladium, iridium, rhodium, osmium, and ruthenium.
One can use lead, bismuth, nickel sulphide and tin as a collecting reagent. The most popular procedure is the lead method and will be the focus of this paper. There are benefits and drawbacks to each procedure. The use of lead has environmental & health consequences, and therefore due diligence should be used with respect to the assayer exposure to the lead. There are health consequences of exposure to high lead levels and regular blood tests and consultations with a physician should be required for the assayer. There also should be proper protective personal gear provided and worn, such as respirator that has been fit tested. The filters on the respirator should be changed on a regular basis.

Eye protection, gloves and coveralls should be worn at all times. Hygiene should a top priority for everyone.

The equipment such as the loading forks, pouring tongs should be supplied as one is dealing with extremely high temperatures.


The fume hoods and the furnace should also have regular maintenance performed on them by professional to make sure that it is performing within the operating parameters.
The disposable of the used lead cupels should done by certified environmental company with accordance of the environmental laws set out by the regulations of the governing body. As a consequence of the concerns of the effects of lead, bismuth is starting to be used more now because it has no negative health or environmental effects, but it is generally more expensive.
There are five steps in fire assaying.
- Fluxing and fusion of the sample.
- Separation of the slag from the button.
- Cupellation.
- Parting
- Weighing finish / Instrumental finish
A fire assayer must have the ability to set up the process of weighing, fluxing, fusing, pouring, cupellation, and parting in an orderly, organize fashion. The samples must maintain in order from start to finish. It is important to invest some time to set up a structure of how to proceed from start to finish before beginning the process.
One should study each sample visually before weighing. Each sample is unique and one can learn from visually inspecting the sample and from possible past assays done on that sample, the amount of the sample that is required. This determination of the weight of the sample helps produce a normal size button. A button can have a range between 20 and 40 grams. The weight of the sample should be recorded for the finishing calculation. One thing that an assayer should keep in mind is that the finer the ore is pulverized and crushed, the better and uniform the results. Though one should be careful as some materials that contain gold and that are crushed and pulverized can lead to smearing of the gold particles, which can cause faulty results. One should try to weigh a uniform sample, large lumps should be avoided when weighing. One should keep in mind whether duplicate assays are going to be performed as to save enough samples for those assays. One can weigh between 10 – 30 grams of the sample. Samples can come in several different colours, one can used the colour and how the sample sparkles to determine the how much of the sample to weigh. If there have been others assay done on the sample, for other elements, one can use that data as well to determine the amount needed to be weighed. The darker the sample usually would indicate that a weighted of 10 grams would work. A white and dull appearing sample would indicate a larger weight would work better.
A Picture of weighed samples.

Fluxing and Fusion of the Sample
There are different classes of samples based on geology. The rock classes are acidic and basic. Samples high in silicates, aluminates or borates are acidic. Samples high in oxides or carbonates and other metals are basic. The information whether the sample is basic or acid is important because it has a huge effect on the choice of fluxing reagents.
There are many steps to fluxing and the fusion of the sample such as the determination of the correct proportion of the fluxing reagents, the mixing of the flux, the loading in the assay furnace, and the pouring of the fused materials. The fluxing and fusion of the sample is the most difficult aspect and where most mistakes are made. Even the most seasoned fire assayer can be fooled by a sample. One will find that there is always something to learn when it comes to fluxing and fusion of a sample. Fluxes provide the chemical reaction that breaks down the sample that leads to the ability to produce a lead alloy that contains the precious metals. The fluxes provide the metallic lead button, and a slag that dissolves unwanted substances such as silicates and borates of metallic oxides that were contained in the sample and the fluxing reagents. The selection of the flux reagents is extremely important because it can produce an ideal slag, which is critical in the fusion step. An ideal slag is important to get the best possible recovery of the precious metals.
There is a classification of fluxes: basic, acidic and neutral.
Basic Fluxes
Acidic ores needs a basic flux to produce an ideal slag from the resulting fusion and in turn produce the best results. Acidic sample tend to produce thick slag and shots of lead in the fused materials, which will produce faulty results. Sample high in silicates and/or aluminates are the most common that an assayer would deal that typically cause these problems. A way to solve these problems would be to add a basic flux, for example soda ash.
Here is a list of basic fluxes use in fire assay:
Sodium carbonate (Na2CO3) or Soda ash
Sodium carbonate is a powerful basic flux that reacts with silica-based (acidic) minerals to form a fusible sodium silicate.
Na2CO3 + SiO2 = Na2SiO3 + CO2.
There can be problems with soda ash reactions. The CO2 gas that is produced can cause frothing if the temperature is too high. Soda ash is very fluid and can hold finely powdered materials such as carbon (flour) in suspension in the melt which can interfere with the formation of the button in the fusion process. Soda ash can also act as a desulphurizing and oxidizing agent. Soda ash is also very corrosive towards the crucible.
Sodium bicarbonate (NaHCO3) baking soda
This can be used in place of soda ash but it is less common. It has less soda, which is the active ingredient. It loses water and CO2 at low temperatures and can cause losses due to, cross contamination, frothing, and the sample boiling over. The end product of the use of sodium bicarbonate is the same as with soda.
Litharge
(lead oxide – PbO) & Red lead (lead oxide – PbO:Pb2O3 or Pb3O4)
Litharge (PbO) or red Lead (Pb3O4) is a basic flux that has many purposes. It helps to decompose silica to form fusible lead silicate.
PbO + SiO2 = PbSiO3
It supplies the lead to produce the lead button that collects the gold and silver.
PbO: 2PbO + C(flour) = 2Pb + CO2.
It also dissolves difficult-to-fuse metal oxides for example Copper. One should practice some caution because excessive litharge corrodes the crucible and destroys the furnace floor if there is a spill or boil over.
Red lead acts as an oxidizing agent as it decomposes to PbO and gaseous O2 on heating as oppose to CO2 and is sometimes preferred to litharge.
2Pb3O4 = 6PbO + O2.
Hematite (iron oxide – Fe2O3)
Hematite is sometimes used as to replace litharge. It is combination of a basic flux and an oxidizing reagent. Ferric oxide is very difficult to fuse but is reduced to a more easily fused ferrous state during the fusion. It then combines with silica to yield ferrous silicate. Manganese oxides act similarly to iron oxides. These oxides act as oxidizing agents by consuming carbon (flour).
2 Fe2O3 + C = 4FeO + CO2
2 FeO + SiO2 = 2FeOSiO2
Lime (CaO)
Lime also known as the mineral limestone (CaCO3). It is a powerful base for fluxing the silica. On heating, limestone loses CO2 and reacts with silica and produces a fusible silicate. Calcium also is found with magnesium in a carbonate mineral called dolomite (CaMg(CO3)2). Magnesium behaves very similarly to calcium in a fusion.
Acid Fluxes
Basic sample require an acidic flux to produce an ideal slag. An example of a basic sample would be limestone/dolomite samples. One can performed a quick test to ensure that a sample is limestone by adding a couple drops of HCl acid. The samples will fizzes which is the CO2 that is produced.
Borax Glass (sodium tetraborate – Na2B4O7)
Borax glass is a strong acid flux and it is used in almost all fusions. Borax glass can act as an acid and base. Borax has several helpful properties in the fusion process. It lowers the fusion temperature for the formation of slags. It is capable of fluxing most metal oxides. It also increases the fluidity of the slag, which can help with difficult pours. The addition of too much borax can result in a very tough slag that is hard to separate from the button. The slag sticks to the button and results in a loss of precious metals and low results. One also should decrease the amount of borax when dealing with a high grade copper sample because the bead can be absorbed in the cupel during the cupellation. If one can not decrease the amount of borax in a flux to resolve the problem, one can add a basic flux which can provide a solution to the problem.
Hydrated borax (Na2B4O7 x 10H2O) it should be avoid because it often produces boil-overs because the salt in the borax loses water and causes swelling of the charge during the fusion.
Silica (SiO2)
Silica is the strongest acidic fluxing reagent. It combines with the metal oxides to form silicates. The silicates are the foundation of all slags. It is a fine powder and is added to samples which are basic to help protect the crucible in the fusion process. When too much of silica is in the flux, it can reduce recovery of the precious metals because of the precious metals that can be lost in to the slag or the formation of matte. Powdered glass can be used as a substitute. The amount of SiO2 varies in glass and therefore may cause a problem with an assayer ability to perform reproducible assays.
Neutral Fluxes and Neutral Sample
Neutral samples are ores that do not reduce or oxidize and are neither acidic nor basic. These ores are fluxed with a standard premixed flux. Neutral fluxes are used mainly to help control the fluidity of thick slag.
Fluorspar (calcium fluoride – CaF2)
It is used as a flux in fire assaying because it increases the fluidity and helps with difficult pours. It melts at a high temperature and is very fluid. It suspends the infusible particles without lowering the fluidity of the slag. It is mainly used in fluxing ores containing calcium phosphate (bone ash), barites, gypsum, and infusible silicates. Fluorspar melts and remains unchanged in the slag. Fluorspar gives a stony appearance to the slag.
Salt (sodium chloride – NaCl)
Slat is added as a cover to fusions to protect the flux from air oxidation. It is very fluid and it is used to wash down particles of the metal that stick to the sides of the crucible. It is unchanged during the fusion.
Cryolite (AlNa3F6)
Cryolite is used as a flux to aid in the fluxing of high Al2O3 ores such as clays and occasionally in the melting of bullion.
Reducing Fluxing Reagents
Reducing samples are ores that contain sulphides, arsenides and carbonaceous substances. One wants to reduce the litharge to lead, PbO → Pb. The reducing power is the amount of lead that one gram of an ore or reducing agent will reduce.
Flour
Flour is the most commonly used reducing agent. It is used in samples that have little or no sulphites in order to reduce the litharge to molten lead for the collection of gold and silver. Carbon is the active ingredient in flour. The flour that used is ordinary wheat flour. It is has several advantages. It is cheap and it is readily available. It also is very fine and mixes well with the ores and fluxes. One thing that one should consider when using flour is that flour has varying moisture content. Generally, 1 gram of flour will reduce 11 grams of Pb.
Tartar (potassium bitartrate – KHC4H4O6)
This is cream of tartar or potassium bitartrate. The carbon and CO act as the reducing agents. It has less than half of a reducing power then flour, about 2.5 grams is required to reduce 11 grams of Pb.
Charcoal
Charcoal is about 90% carbon. It has been used in the past but it is not as common now. It reacts with metal oxides by the production of CO2 at lower temperatures and CO at higher temperatures. It has twice the reducing power as flour, but the quality can vary.
Oxidizing Fluxing Reagents
The oxidizing power in fire assay is the ability of a substance to give up its oxygen.
Nitre (potassium nitrate – KNO3)
Nitre is an important and powerful oxidizing agent. It oxides sulphur and many metals by releasing its oxygen. For example it is used quite regularly in the assaying of zinc, lead and copper. It oxidizes sulphides to sulphates, arsenide’s to arsenates and most metals to their oxides. It is used mainly in high sulphides samples to control the size of the button. If large amounts of nitre are needed to fuse a sample one must pay attention to the temperature of the charge to prevent boil-overs. One should try to heat the charge in evenly and gradually. Nitre is also known as desulphurizing agent. In the process of fusion, it decomposes in 2 stages. The first stage is to KNO2 and then to K2O at a higher temperature:
2KNO3 = 2KNO2 + O2
2KNO2 = K2O + 2NO + O
Red Lead (lead oxide – PbO:Pb2O3 or Pb3O4) can be used for its oxidation action in a fusion and it is used to replace litharge. It is used in a limited way because it can produce too much lead in the fusion mixture. The oxidizing power of red lead comes from the release of O2 when it is heated. The PbO that is produced is further reduced to Pb by sulphides in the sample.
Hot air is also an oxidizing agent. The oxygen that is in the air is the oxidizing agent, which is similar to red lead.
Desulfurizing Reagents
Desulphurizing reagents are substances can attract sulphur and pull the sulphur away from the other compounds.
Inquartation
In order for the fire assay of precious metals there needs to be a collecting agent that assists in the parting process. A small amount of silver is usually added to the crucible during the mixing of the fluxes. There has to be a ratio of 3Ag:1Au for the parting process to proceed and for the parting process to work properly. There are different ratios required for gravimetric and instrumental finishes.
The Fluxing and Fusion Process
Once the sample is weighed and the assayer has organized as how to proceed in an orderly manner. The assayer should inspect and arrange the crucible in a fume hood. After the crucibles have been set up to the assayer liking, the assayer should determine if the sample needs to have silver added to the flux, to produce the right ratio of Ag:Au, if so silver should be added. The assayer should have by now determined the correct amount of fluxing reagents.
A standard flux charge depending on the sample is as follows:
One can use a 60 gram or 40 gram crucibles. The crucible should hold the charge with room to spare, as this will prevent boil overs.
60 grams of litharge- 2 Tablespoons
30 grams of soda ash – 1 Tablespoon
5 grams of borax – 1 teaspoon
If the sample is high in copper, one can use 2 grams of borax.
If the sample is high in zinc and/or pyrite one tends to increase the amount of borax to 10 grams.
4 grams of silica – 1 teaspoon.
If the sample is high in copper, one can use 8 grams of silica.
If the sample is high in zinc and/or pyrite one tends to increase the silica to 12 grams.
Depending on the sample the assayer one would add either nitre, flour or perhaps nothing at all to the crucible at this stage. This is the most difficult decision, but with experience the assayer will gain the confidence to make the right choice. If in doubt, one can always ask a more experience assayer for their advice.
5 grams of nitre – 1 teaspoon
4 grams of flour – 1 teaspoon

One should then pour the sample in the crucible making sure the entire sample enters the crucible. One then should mix the charge as thoroughly as possible. The mixture of ore and reagents should ensure that each particle of ore is in contact with litharge and reducing agents.

Place the mixed charges into a furnace between 1900 – 2000°F. As the mixture heats up, litharge is reduced to lead by the carbon or sulphides of the charge. The sample minerals fuse and the shots of lead then take up the freed gold and silver from the surrounding particles of ore. This produces an alloy with the lead and the precious metals. At the same temperature, the borax of the charge begins to melt and forms fusible compounds with some of the bases of the flux. The fusion usually takes 45 minutes at a temperature between of 1950 to 2000 °F. Lead oxide and silica combine and from this point the slag begins to form rapidly. The slag remains vicious until the ore particles are thoroughly decomposed and every particle of gold and silver has been taken up by the suspended molten lead. The slag becomes thoroughly fluid, and the lead falls through the slag, collect at the bottom of the crucible. Samples that required a high nitre flux or gas-forming substances such as carbonates, close attention should be given to the crucibles because of possibility of boil overs. If there is evidence of frothing, the door should be opened to cool the fusion down.
The molten mixture is poured into a metal mould where the Pb collects beneath the slag as a small cone. Pouring moulds are conical iron depressions.

When the assayer is pouring the flux charge of molten mixture in to the mould, the assayer should pay attention to the way the mixture pours and if there is any evidence of shots of lead. This can be good indication of if there will be problems and undesirable products.

Separating the Slag from the Button
When the slag is cooled, the slag is separated from the Pb button by pounding it off with a hammer and then cubing the Pb button so it can be easily picked up. The lead button should be bright, it should separate from the slag easily and there should be no evidence of matte or speiss on the top surface of the button. The Pb buttons should be between 15 to 35 grams. One should keep a record of past sample fluxing history as to change and improve on the fluxing of difficult samples. This can help to attain correct button size, poor slag/ button separation, matte or speiss and other problems. After the fusion material is poured into mould and cooled, the lead button is separated from the slag by hammering the slag off the button.
There are two undesirable products matte and speiss that are occasionally obtained. There can be a third product if salt is used or if nitre is used in the assay. The third product will be found on the top of the solidified slag.
Mattes
Matte is an artificial sulphide of metals found in the ore and/or flux.
It is formed in the nitre fusion of sulphide ore when the charge is too acid.
It is found just above the lead button. It may be quite thick, or it may appear simply as a granular coating on the upper surface of the lead button. It is usually blue- grey in colour, resembles galena and is very brittle. The matte always carries some of the gold and silver. It is usually broken off and lost in the slag during deslagging of the lead button leading to low results. If it occurs, the assay must be repeated with more basic fluxes and less acid fluxes.
Speiss
In the fire assay speiss is usually a combination of Fe & As. Speiss is formed when the iron method is used on ores containing arsenic. It is a hard, fairly tough, tin-white substance found on the top of the lead. If a small amount of arsenic is present, the speiss will form a little button on the top of the lead. If there is lot arsenic is present, the speiss may cover the entire top of the lead. Speiss contains Au & Ag, and may be cupelled along with the lead if there is less than a gram of speiss. Larger amounts are difficult to deal with and the sample should be assayed by another method.
Lead Shot in the Slag
This is usually caused by the temperature being too low or too little silica in the charge. Should shotting occur, increase the amount of silica and litharge and re-fuse at a higher temperature.
What are the Properties of an Ideal Slag
One must try to produce an ideal slag because it will help with the recovery of the precious metals. The best slags are a balance between acidic and basic properties. Slags are classified according to the proportion of oxygen in the acid to the oxygen in the base. A mono-silicate slag has equal amounts of oxygen in each whereas bi-silicate slags have twice as much oxygen in the acid as in the base. The best slags are those that range between a subsilicate and a bisilicate. The different fluxing reagents all have a role in producing an ideal slag. The fluxing and fusion procedures require that the fluxes balanced each other. It should be glassy and homogeneous in colour and texture. Streaky slags are a sign of an imperfect fusion. A sample that indicates high S, As, Sb, Cu or any other base metals requires a knowledge of how to proceed with the fluxes that is gain by experience and asking question from those assayer whose do have the experience.
Here is a list of some common slags.
Various Greens = Ferrous Silicates
Brick red = Copper
Opaque Milky White = Ca, Mg, Al, Zn, Ba – Limestone sample will produce a greenish, opaque and/or streaked slag.
Black = Fe & Mn
Blue = Co
Purple to light Pink = Mn
Greenish white = very high sulphide.

Fusion Tips
Place crucibles in sequence after pouring. A rack should be set aside to dispose the hot crucibles.
Set aside crucibles with unusual pours or lead buttons. A history of difficult or problematic samples should be kept.
Ideal Lead Button Size
The size of button to aim for is a range from 20 to 35 grams.

Sample Roasting
This is a technique used to handle difficult samples. Minerals decomposed in this way include sulphides, arsenides, antimonides and tellurides. A successful roasting can render samples amenable to standard flux mixes. Weigh 50 grams of a sample into a shallow dish and place it into an open furnace at 100 °C. Raise the temperature slowly to 600’C with careful stirring. When no fumes are seen, raise the temperature to 700’C for 20 minutes. The S, As, Sb, and Te are lost as volatile species. Their elimination simplifies the fluxing and prevents the formation of speiss or matte deposits. Care must be taken not to fuse galena because Ag is lost during this process
Cupellation
Cupellation separates the gold and silver from the lead. This consists of an oxidizing fusion in a porous vessel called a cupel. The objective of cupellation is the conversion of Pb and other base metals such as Cu that are in the lead button into oxides. The oxides are separated from the Au, Ag and other precious metals because they do not oxidize under cupellation conditions.

One should check furnace temperature and inspect cupels for smoothness & foreign matter. Blow debris from cupel with low pressure air canister. Place empty cupels in furnace for 10 minutes in order to expel all the moisture and organic matter. The pounded lead buttons are placed into the cupel in the furnace and the lead is oxidized to PbO, which is absorbed into the cupel. If the cupels are thoroughly heated, the lead will melt at once and become covered with a dark scum. If the temperature is correct this will disappear within minutes. The lead will become bright once this has happened and it is said that the button has opened up or uncovered. This signifies that the lead has begun to oxidize. Close vent & door for 5 minutes to make sure that all the buttons have opened up. The door is opened slightly to admit a supply of air to promote oxidation of the lead. The air oxidizes the Pb to PbO, 98.5% soaks into the porous surface of the cupel and about 1.5% volatilizes. Continue to cupel for 35 – 40 minutes. If the temperature of the furnace becomes to low when the door is opened to allow the supply of air for the oxidation, the litharge will not be absorbed into the cupel and the lead will solidify. When this occurs, the button is said to have frozen. The assay should be rejected, as the results are usually low. One can try to bring them to the driving temperature with a popsicles stick, this sometimes works. The oxidation proceeds at a rate of about 1 g of lead per minute so 30 gram buttons should take about 30 minutes for all of the lead to be oxidized. The Au, Ag and the other precious metals do not oxidized and are left as a small bead in the bottom of the cupel.

The precious metals in the original sample have been concentrated into the 2 or 3 mg bead. If the fluxing, fusion and cupellation are done properly, the Au, Pt, Pd found in the bead will represent well over 99% of that in the sample. There are small losses to slag and the cupel but they are not usually significant. Silver losses are usually more significant and have to be taken into account when reporting Ag analysis based on fire assay work. Silver losses are determined by the cupellation of a known amount of Ag using the same mass as is expected in the sample. The weigh difference of the control silver before and after cupellation is added to the Ag of the sample as a correction.
There are losses in cupellation and the amount depends on factors such as the nature and shape of the cupel, the temperature of cupellation, the proportion of lead and silver, the amount of the impurities and the draft through the furnace. The losses of the precious metals are from sprouting, absorption of bouillon by the cupel, oxidation and absorption of gold and silver and volatilization of silver and gold. With good cupellation the losses of precious metals is due mainly to oxidation and absorption of the oxides. The small losses are due to volatilization. Silver is more readily oxidized than gold and the bead is higher percentage of silver. The most important single factor relative to cupel loss is the temperature. The cupellation should always be conducted at the lowest practicable temperature. There several things that can make the cupellation easier. After cupels are driving, open vent and furnace door to speed up the process. Do not overload Pb buttons directly from the moulds to the cupels. For large beads that are high in Ag, one should slowly remove from the furnace or cover the cupel with an empty hot one to prevent sprouting. Increased time is required when loading buttons into cold cupels.
Indications of Metals Present
- Zinc burns as brilliant greenish white flame and emits dense white fumes.
- Tin oxidizes quickly and forms SnO2 which covers the lead with infusible yellow lava fragments and stops the cupellation.
- Iron gives brown or black lava fragments, as does cobalt and manganese. Small amounts of iron oxide will stain the cupel dark red.
- Nickel will deposit dark green lava fragments and stain the cupel green. Large amount of nickel will cause the button to freeze.
- Copper is absorbed in the cupel and gives the cupel a stain that ranges from dirty green almost to black according to the amount present.

Parting
Parting is the process of dissolving the Ag in the bead from the cupellation to leave the Au behind for weighing or to be read on the AAS.
If Ag is to be determined gravimetrically as well, the bead is weighed before parting. The very small beads are weighed using sensitive micro-balances.
Parting Solutions
Nitric Acid (HNO3) – Dissolves Ag and Pt group metals & leaves the gold behind.
Sulphuric Acid (H2SO4) – dissolves Ag ; leaves the Pt group metals & gold behind.
Hydrochloric acid (HCl) – In the AA finish, HCl is added after the Ag is dissolved to form aqua regia (3:1 HCl to HNO3) . Aqua regia dissolves the gold
Ammonia (NH4OH) – used to dissolve any possible silver chloride which may have precipitated out.
Nitric acid is almost used all the time but sulphuric acid can be used as well. Nitric can not be used unless there at least three times the amount of silver than gold. The idea of parting is to prevent the gold from breaking up into small particles. The gold that remains in one piece is much easier to handle.
Acid Concentrations
Gravimetric finish
6:1 HNO3 for gravimetric finish
3:1 HNO3 for gravimetric finish
1:1 HNO3 for gravimetric finish
1:5.5 H2SO4 for gravimetric determination Of Pt Group metals.
Instrumental finish
0.5 ml HNO3 of concentration for AA finish
1.5 ml of conc. HCl for AA Finish
Gravimetric Finish
Ensure parting cups are clean before using. The assayer should put a flatten bend in hot 6:1 nitric to start, and watch the bead for its reactivity, add stronger acid if it is needed. This should be done on a hot plate and inside a fume hood. If stronger acid is needed it should be hot. One should carefully add and remove the acid with a disposable pipette. To make sure that floaters are fully parted, use glass rod or tap cup to sink to sink them. One can add ammonia rinse solution and to remove any AgCl that is present. There are microbalances that can go down to 0.1 mircograms which allows the gravimetric detection limit to improve over the gold balances in the pasts. Handling the very small beads can be very difficult, so gravimetric finish is reserved for higher grade samples.
Rinsing
After parting is completed, one should rinse the bead. One can use rinse with 14:1 NH4OH to remove any residual AgCl or use distilled water.
Drying & Annealing
One should allow any moisture to dry because the Au can be lost due to spiting. One can do this by placing the parting cup on a hot plate to dry. Annealing the Au should be done over a flame. This turns the Au from black to gold and hardens it and causes the particles to stick together.
Annealing Tips
Inspect and remove foreign particles. When using a flame for the purposes of annealing, one should heat the bottom of the parting cup until it is red.
Fire Assaying Problems
The bead should not have a gold/ brass color, if it does, it means the bead is not fully parted because of the low Ag:Au ratio. For gravimetric finish one should inspect the parting cup to make sure that it is clean and weigh the sample for the same length of time as it takes to tare the balance. Flouring is when the bead breaks up during parting due because of several reasons because of incorrect Ag:Au ratio, cold parting acid, and impurities.
Instrumental Finish
One should first pick and flatten the beads and put them into 16 x 125 mm disposable glass test tube. One should ensure water bath is a minimum of 75 °C. Another option is the use of fine sand. The sand gets far hotter than a water bath

The assayer should add .5 ml of concentrated HNO3 from a calibrated dispenser and place rack in hot water bath for 15 minutes. After 15 minutes the assayer should confirm by visually inspecting the test tubes for the complete silver digestion. After the assayer confirms that the silver bead is digested, one can proceed to digest the gold by dispensing 1.5 mL of concentrated HCl from a calibrated dispenser into the test tube.

Once both the acids are added one should again inspect each of the test tube for complete digestion of the gold. The final steps are to dilute to the final volume and shake the test tube to mix the solution thoroughly. The addition of the acids should be done inside of a fume hood because of the fumes that are produced.
Bead parting tips for readings done on an AAS or ICP OES/MS are as the follows. One should check that each tube has a bead in it. One should also check that the acids are dispensed correctly. The aqua regia should be an orange/yellow colour. One can look for the AgCl precipitate in each test tube. For the measurement of gold by AAS or ICP/OES measurement, the entire bead must be dissolved so the instrument can measure the solution concentration. The instruments determined the concentrations by aspiration the sample into the calibrated instrument. This instrumental method is much more common for finishing then measuring with the gravimetric technique. The instruments are capable of a much lower detection limits. Labs can measure a 1 or 2 ppb detection limits using AAS, ICP OES and ICP MS.
Fire Assaying Platinum group Elements (PGE’s)
The standard lead collection scheme used for gold gives good recovery for platinum and palladium as well. They are soluble in aqua regia and can be measured with gold in a multi element instrument such as ICP/OES or ICP/MS. The detection limits are low ppb.
The elements osmium, ruthenium and iridium are lost during the standard lead assay and cupellation process. Instead, they have been determined using the nickel sulphide collection technique. The nickel sulphide technique uses a 10 g of sample, 1 g powdered Ni, .8 g S, 12g soda ash, and 24 g borax.
The mixture is fused at 1010 °C for 1 hr. Then it is cooled and the assayer separates the NiS matte from the slag and pulverizes the NiS matte. The pulverized matte is dissolved in concentrated HCl. A filter is used to collect the PGE sulphides . Once the PGE’s are collect, they are dissolved in aqua regia, dilute, and measure on ICO/OES or ICP/MS. The Ni powder is very toxic and the concentration HCl digestion step can cause serious fume problems and are need to be done with a good exhaust system. The method is expensive, and the PGE’s that are assayed by this method are rare.
Glossary of Fire Assay Terms
- Annealing – the heating of gold after parting to change its colour from black to gold.
- Bead – Particle of gold and silver that is obtained at the end of the cupellation process.
- Borax – An acid flux.
- Crucible – A bone ash vessel in which the sample and fluxes are melted.
- Cupel – A shallow cup which the lead button is oxidized during cupellation.
- Cupellation – Process of burning off the lead button.
- Feathers – crystals of lead oxide on the edge of the cupel. Indicates that the cupellation worked as it should.
- Flux – reagent mixed with a sample that aids in the fusion process.
- Freezing of the cupel – A condition in which solid lead oxide or other solids cover the surface of the molten lead in a cupel.
- Fusion – Process of heating a charge in a crucible to cause reactions and produce a molten liquid.
- Inquartation – The addition of silver to ensure that the bead will part.
- Lead button – Metallic lead produced during a fusion and becomes solid in the mould.
- Lime – A basic flux.
- Litharge – Lead oxide, a basic flux and a source of lead used in fire assay.
- Microbalance – A very sensitive balance that weighs beads.
- Mould – An iron cone shaped vessel, which fused materials are poured into.
- Muffle – A chamber in a furnace in which fusion and cupellation are carried out.
- Opening of the cupel – At the beginning of cupellation when the surface of the lead oxide melts.
- Ore – A natural material that came from the earth, that is to be assayed.
- Oxidizing agent – An ore or a reagent that supplies oxygen for reactions during the fusion.
- Parting – Method of separating gold and silver by dissolving the silver.
- Parting acid – Nitric, Hydrochloric and Sulphuric acids.
- Parting cup – A porcelain vessel which parting, rinsing and annealing of the gold takes place.
- Reducing agent – An ore or reagent that accepts oxygen during the fusion which helps in the formation of the metallic lead.
- Reducing Power – The ratio of lead reduced during the fusion to the weight of the reducing agent added.
- Scorification – An oxidizing fusion of a sample with lead and borax in a shallow dish.
- Slag – A fused mixture of non-metallic sample ingredients and fluxes, usually contains silicates.
- Soda ash – Sodium carbonate, which is a basic flux.
References:
Edward E. Bugbee, A Textbook of Fire Assaying, Legend Metallurgical Laboratory, Inc, 3rd edition.