Table of Contents
Acid ferric sulfate solution has been used for heap and dump leaching of low-grade chalcopyrite ores and is being considered as a possible lixiviant for the hydrometallurgical processing of copper sulfide concentrates. For many years, researchers have attempted to explain the leaching behavior of chalcopyrite, since the reaction rate kinetics are extremely slow and seem to be significantly influenced by the nature of the reaction product.
If the reaction rate for ferric sulfate leaching of chalcopyrite is limited by electron transport in the protective elemental sulfur product layer, modification of this reaction product layer to increase its conductivity should increase the rate of the leaching reaction. To obtain an increase in the electrical conductivity of the sulfur layer, the principles of solid-state chemistry as applied to the conductivity of insulators and semiconductors may be considered.
Experimental
Experiments were designed to study the effects of particulate carbon additions on ferric sulfate leaching of chalcopyrite. To obtain contact between chalcopyrite and carbon particles, an experimental technique was used which involved intimate mixing of the particles and compression of the mixture to form a CuFeS2/C pellet. Generally the pellet was broken into aggregates. The ferric sulfate leaching of CuFeS2/C aggregates was carried out under atmospheric pressure. The reaction rates were followed by copper analysis of solution samples taken at timed intervals.


Apparatus and Procedure
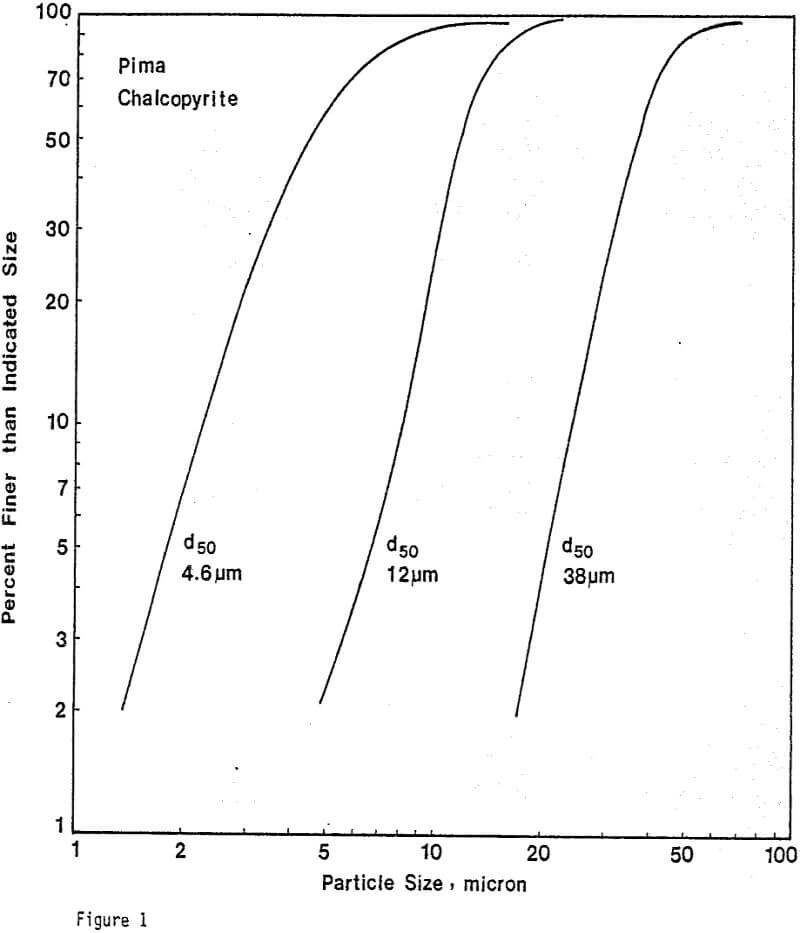
Leaching Experiments. Chalcopyrite and carbon particles were mixed thoroughly and pressed into pellets of appropriate size. The diameter of the CuFeS2/C pellets was 1.3 cm, and the thickness of the pellets depended upon the amount of sample used. Most samples were 0.24 cm thick. The pellets were prepared at 20,000 lb. The compression and porosity of the pellet depend on the particle size and the proportion of chalcopyrite to carbon. Preparation of CuFeS2/C aggregates from 38-micron chalcopyrite particles results in significant breakage of these particles, particularly for small additions of carbon. Generally, CuFeS2/C aggregates prepared from fine chalcopyrite particles are more compact and exhibit greater structural integrity.
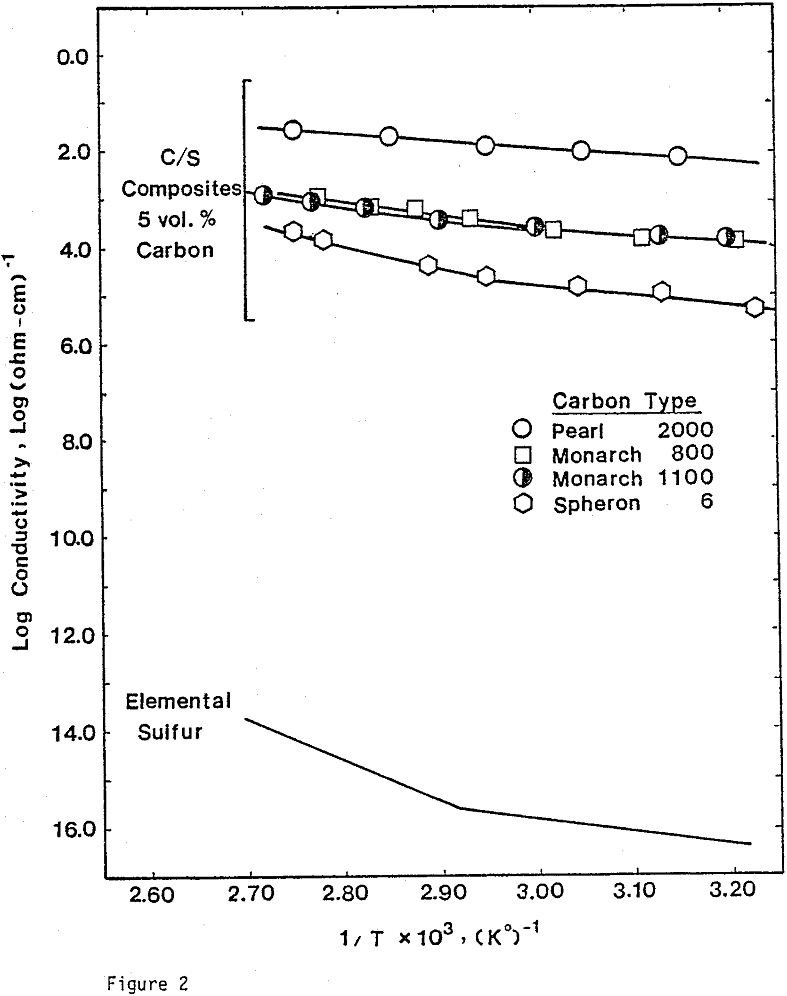
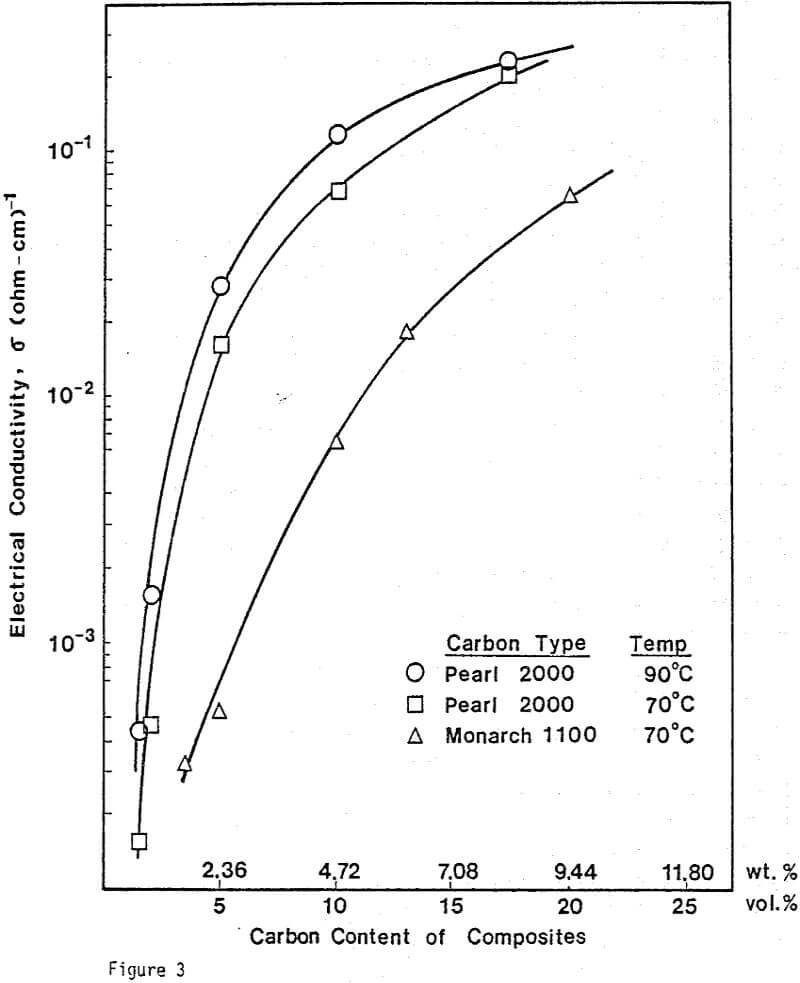
Electrical Conductivity Measurements. To determine the conductivity of C/S composites, carbon and sulfur of 99.98% purity were mixed and pressed into pellets. The size of the pellets was 1.27 cm in diameter and 0.19 cm thick. A wire-wound resistance furnace connected to a temperature controller was used for the conductivity measurement. A three-point electrode system was used to prevent surface leakage. Bulk conductivities were measured under a helium atmosphere.
Experimental Results
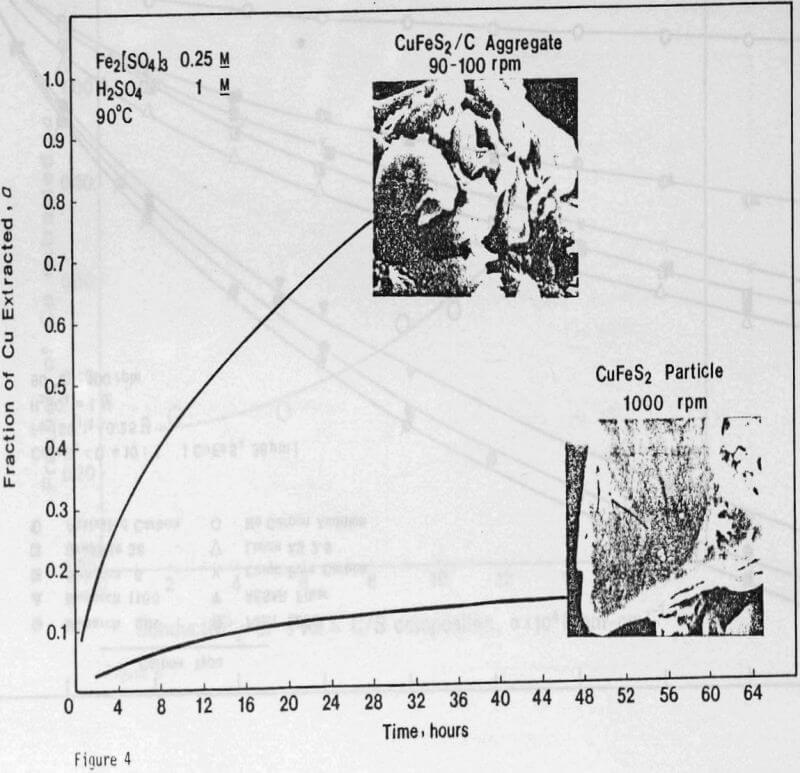
The formation of a dense and tenacious sulfur layer passivates the reaction and slows the leaching rate to unacceptably low levels. The addition of carbon particles reduces passivation and increases the leaching rate.
Nine different types of carbon have been evaluated in leaching studies and electrical conductivity measurements. It seems that the former size is a measure of carbon aggregates which can become dispersed depending on the chemical environment. For example, the C/S composites exhibit good dispersion-characteristics. The extent of dispersion of carbon in liquid sulfur is so great that individual carbon particles are difficult to identify even by Scanning Electronic Microscopy at a magnification of 4000.

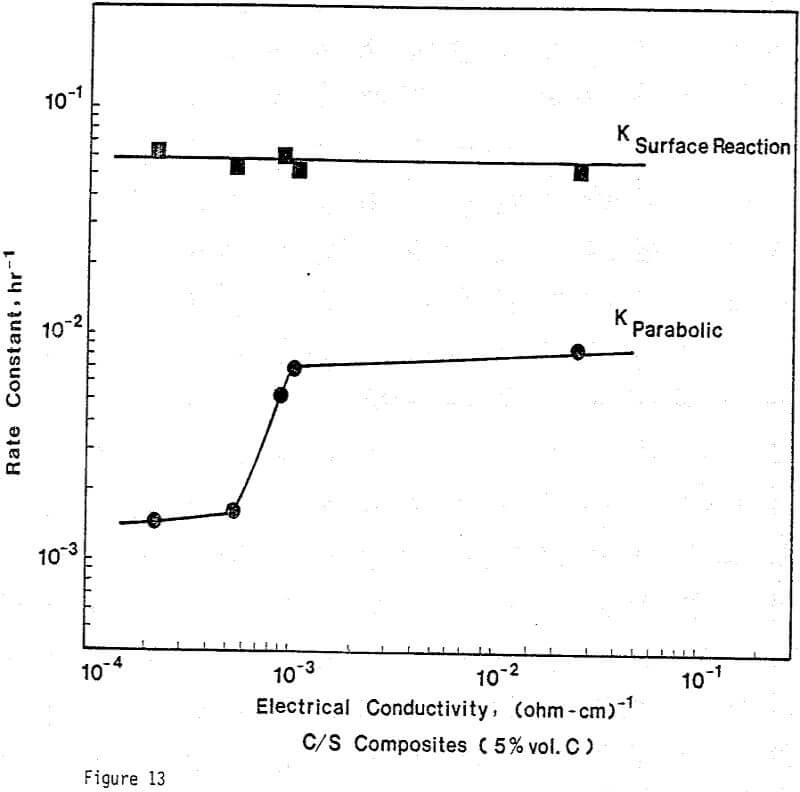
Electrical Conductivity of Carbon/Sulfur Composites
From previous study, it appears that the leaching kinetics of chalcopyrite in ferric sulfate solution is limited by electron transport through the sulfur product layer. The electrical conductivity of the elemental sulfur product layer as estimated from the leaching response of chalcopyrite particles, teσ = 7.6×10 -13 (ohm-cm)-¹, compares favorably with the electrical conductivity of pure crystalline orthorhombic sulfur at 90°C, teσ = 10 -13 (ohm-cm)-¹, reported in the literature. As noted previously, the electrical conductivity of an insulator phase may well be increased by the addition of dispersed particles imbedded in the insulator phase.. The change in conductivity of the matrix material is due to the emission of electrons from, the dispersed particles into the insulating matrix phase.
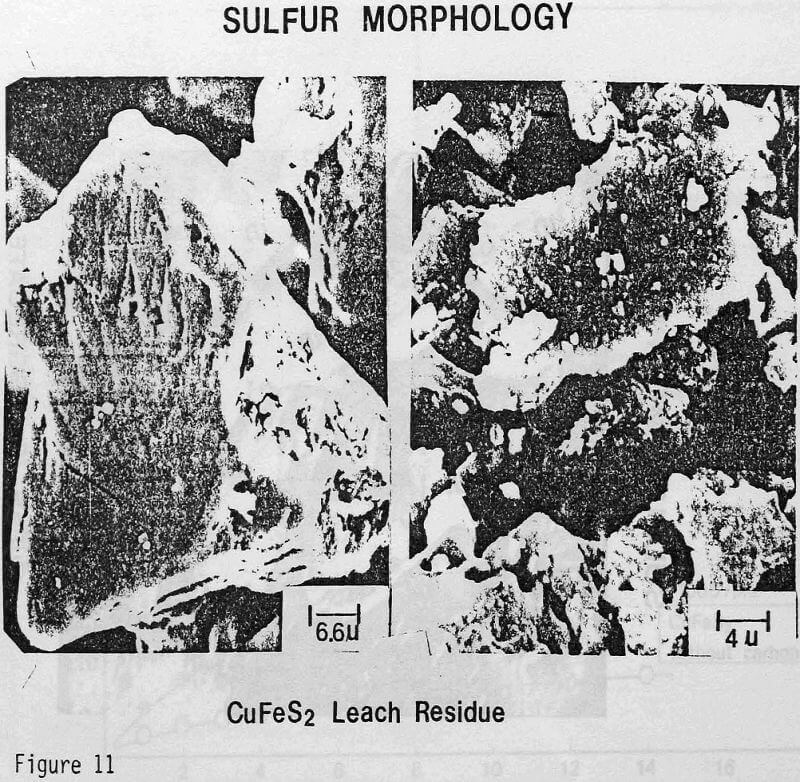
ignificantly faster ferric sulfate leaching kinetics are achieved for CuFeS2/C particulate aggregates than for suspended CuFeS2 particles without carbon addition. The rate of ferric sulfate leaching of CuFeS2/C aggregates at low stirring speed is much greater than the rate of leaching of suspended chalcopyrite particles at high speed . In fact, high stirring speeds for aggregate leaching appear to disrupt the CuFeS2/C aggregates and the extent of reaction is reduced. The nature of the elemental sulfur product layer in the case of aggregate leaching differs significantly from the sulfur structure that forms in the absence of carbon.
Wetting Characteristics of Elemental Sulfur
The sulfur product formed from CuFeS2/C aggregates has been found to have a more botryoidal, less protective character. The inability to identify carbon particles by microscopic examination of C/S composites suggests that excellent dispersion of carbon agglomerates occurs in liquid sulfur. The carbon particles at the chalcopyrite surface may act as nuclei for sulfur growth during the leaching reaction and contribute to the nonuniform, less protective nature of the product layer. In this regard, the wetting of carbon and chalcopyrite by liquid sulfur has been studied to aid in the characterization of the reaction product.
These experiments demonstrate that elemental sulfur tends to completely wet a carbon surface as well as a gold surface. Somewhat surprisingly, such is not the case for a chalcopyrite surface. It seems that the wetting of the carbon particles by the elemental sulfur reaction product is an important factor which ensures good dispersion characteristics.
Discussion of Reaction Mechanism
The experimental results show that enhanced reaction kinetics in the ferric sulfate leaching of CuFeS2/C aggregates can be expected under certain circumstances. But the kinetic aspects of this reaction are complicated, more than one rate process may be involved. The reaction mechanism will be discussed in detail in another publication. A reaction mechanism summary follows.