 The purpose of the present investigation was to study the possible role of Fe3+ ions in the flotation of sulfide minerals, specifically on activated sphalerite and chalcopyrite.
The purpose of the present investigation was to study the possible role of Fe3+ ions in the flotation of sulfide minerals, specifically on activated sphalerite and chalcopyrite.
Experimental
Sample: Specimen-grade Chalcopyrite & sphalerite was obtained from the Ward’s Natural Science Est., Inc. It contains 0.035% Fe, 0.012% Cu and <0.001% Pb by weight, and has a resistivity of 101° ohm-cm.
Reagents: All the solutions used were prepared in double-distilled deionized water using reagent grade chemicals, with the following compositions:
pH 2.0 buffer: 0.01 M HCl and 0.05 M KCl
pH 4.6 buffer: 0.05 M HAc and 0.05 M NaAc
pH 6.8 buffer: 0.05 M NaH2PO4 and 0.025 M NaOH
Ferric ion solutions were prepared by dissolving FeCl3 in pH 2.0 buffer solution. Xanthate solutions were prepared by dissolving freshly purified potassium ethyl xanthate (KEX) in deoxygenated double-distilled deionized water. All of the solutions were deoxygenated before use.
Apparatus and Procedure
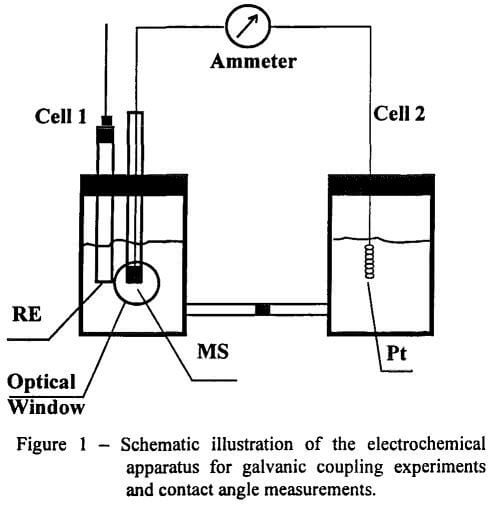
Electrochemical Experiment: Figure 1 shows the galvanic coupling cell designed for contact angle measurements. The cell consisted of two compartments connected by a salt bridge. The working electrode was placed in one compartment (Cell 1), while a platinum electrode was placed in the other compartment (Cell 2). The two electrodes were galvanically connected with each other. Galvanic currents were measured by means of a zero-resistance ammeter (Keithley 485 autoranging picoammeter). A standard calomel reference electrode (SCE) was placed in Cell 1 to measure rest potentials. The Cell 1 was equipped with an optical window to enable contact angle measurements. The measured potentials were converted to the standard hydrogen electrode (SHE) scale, taking the potential of the SCE to be 0.245 V against SHE.
Experiments were also conducted in a single-cell, which consists of the standard three-electrode system. These experiments more closely approximate the conditions for the actual conditioning of ore slurries than those conducted in galvanic coupling cell.
Chalcopyrite electrodes were prepared from specimens cut into cylinders of 6 mm in diameter. A copper wire was attached to one end by means of a conducting carbon paste or an indium solder and encapsulated with epoxy. The electrode was mounted in a glass tubing filled with epoxy, leaving one end of the cylindrical electrode exposed for contact with solution. The electrode surface was wet-polished on 600 grit silicon carbide paper before each experiment. After rinsing with double-distilled water, the electrode was placed in a deoxygenated pH 6.8 buffer solution in Cell 1 with the mineral surface facing down.
Due to the insulating nature of sphalerite, an electrode could not be fabricated in the same manner as with chalcopyrite. Therefore, a sphalerite chunk with dimensions of 15 x 10 mm was placed in a platinum hoop (without epoxy) to ensure electrical contact. Before each test, the mineral was first cleaned in chloroform to remove the KEX coating left from the previous experiment, and then treated with cyanide to remove the copper left on the surface. The mineral surface was wet-polished by using 600-grit silicon carbide paper. The sphalerite electrode was then activated at pH 4.6 in 10 -4 M CuSO4 solution for 30 minutes. After rinsing with distilled water, the activated sphalerite was placed in a deoxygenated pH 6.8 buffer solution, and the rest potentials were monitored.
Contact Angle Measurements: As a mean of determining the hydrophobicity of the mineral electrodes used in the electrochemical experiments sphalerite, contact angle measurements were conducted. For chalcopyrite electrode, which was used in such a way that the electrode surface was facing downward, water contact angles were measured using the captive bubble technique with nitrogen bubbles. For sphalerite electrode, a drop of di-iodomethane (CH2I2) was placed on the surface of the sphalerite sample and the contact angle at the three phase contact was measured. The angle was measured through the aqueous phase. This organic liquid is hydrophobic (γLW = 36.8 erg/cm²) (Adamson, 1990; CRC Handbook, 1990-91); therefore, the di-iodomethane contact angle can be used as a measure of the hydrophobicity of the sphalerite sample. Since the specific gravity of this liquid is 3.33, it was convenient to measure the contact angles of the sphalerite sample whose 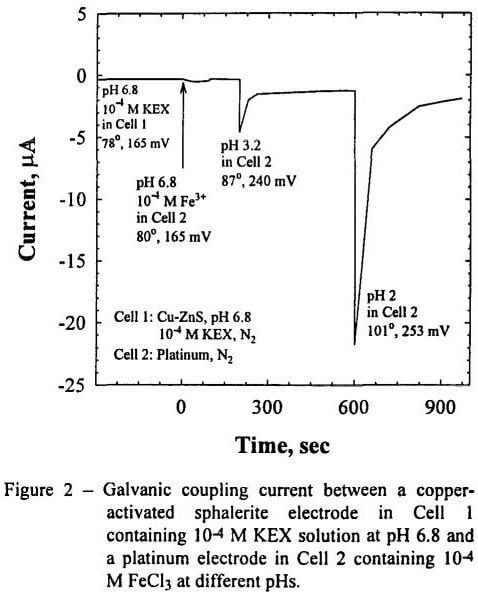 surface of interest was facing upward. Determination of hydrophobicity using the captive bubble or octane-in-water contact angle technique was more difficult because of the buoyancy.
surface of interest was facing upward. Determination of hydrophobicity using the captive bubble or octane-in-water contact angle technique was more difficult because of the buoyancy.
- Galvanic coupling experiments were conducted with a sulfide mineral electrode in Cell 1 containing a xanthate solution and a platinum electrode in Cell 2. The xanthate adsorption, as detected by the changes in contact angle, occurs only when an electron scavenger is present in Cell 2.
- Both oxygen and Fe3+ ions can serve as the scavenger of the electrons generated during the process of xanthate adsorption on sulfide minerals. The results obtained with activated sphalerite and chalcopyrite show that the kinetics of xanthate adsorption is faster when using the Fe3+ ions as the electron scavenger than using oxygen.
- The xanthate adsorption on activated sphalerite is dependent on the activity of free Fe3+ ions present in solution. Thus, the contact angle and the galvanic current increase with decreasing pH of the solution in Cell 2 and with increasing concentration of Fe3+ ions in solution.
- The effects of the Fe3+ ions on the xanthate adsorption on chalcopyrite and activated sphalerite were also studied using a single electrochemical cell, which simulates the conditioner that are commonly used to reagentize ore slurries prior to flotation. The experimental data obtained with the single cell gave the same conclusion as that obtained using the separate electrochemical cells, i.e., xanthate can adsorb on sulfide minerals in the absence of oxygen, provided that Fe3+ ions are present in solution.
- The single-cell experiments show that xanthate can adsorb on a copper-activated sphalerite electrode at pH 6.8 at 10 -4 M FeCl3 in the absence of oxygen, despite the fact that most of the iron are tied-up as Fe(OH)3 at this pH. This finding might be explained by the possibility that Fe(OH)3 precipitate may act as a reservoir for Fe3+ ions during the process of xanthate adsorption on the mineral surface.

