Table of Contents
The Anaconda Minerals Company operates a molybdenite and copper producing mine and mill facility near Tonopah, Nevada. Included with the milling facility is a ferric chloride leach plant for removal of copper mineralization from the molybdenite concentrates. Discussed are the engineering and construction schedules associated with the leach plant. The chemistry associated with the process along with a description of the process flow, equipment, and operating parameters are contained in the paper.
Process Chemistry
The chemistry of the extraction of base metals with chloride oxidants can be viewed at several levels of complexity, much like increasing the power of a microscope to observe finer details. At the least complex level, the extraction of copper and lead from a molybdenite concentrate, as it relates to the Tonopah mineralogy, can be described by the following stoichiometric equations:
Leaching:
4FeCl3 + CuFeS2 → 5FeCl2 + CuCl2 + 2S……………………………………………………..(1)
2FeCl3 + PbS → PbCl2 + 2FeCl2 + S…………………………………………………………….(2)
2FeCl3 + ZnS → ZnCl2 + 2FeCl2 + S…………………………………………………………….(3)
3CuCl2 + CuFeS2 → 4CuCl + FeCl2 + 2S………………………………………………………(4)
2CuCl2 + PbS → PbCl2 + 2CuCl + S……………………………………………………………..(5)
2CuCl2 + ZnS → ZnCl2 + 2CuCl + S……………………………………………………………..(6)
Re-Oxidation:
2FeCl2 + Cl2 → 2FeCl3…………………………………………………………..(7)
CuCl + FeCl3 → CuCl2 + FeCl2………………………………………………..(8)
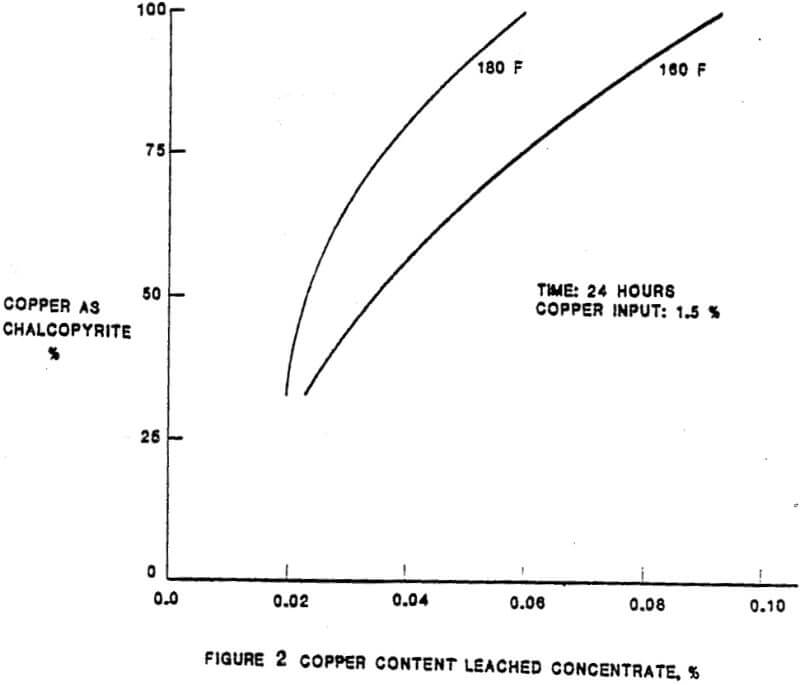
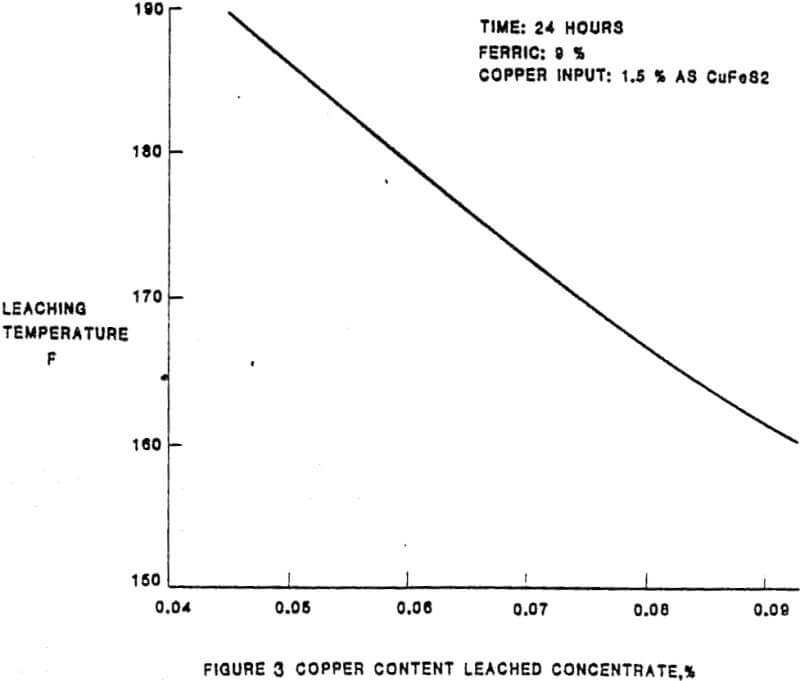
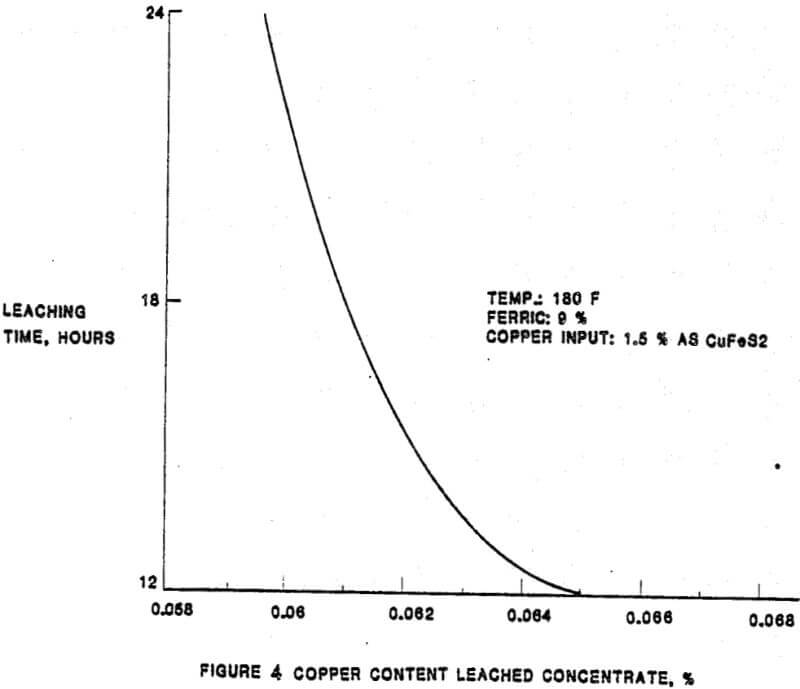
In the solubilization of the copper and lead impurities from the Tonopah concentrate, there are essentially five parameters of importance. The relationship between the extraction of the impurities and the five parameters were established as a result of laboratory test work completed prior and subsequent to the construction of the leach plant. The initial plant results have confirmed the laboratory results. The parameters, in approximate order of importance, are:
– Mineralogy
– Temperature
– Ferric and cupric concentrations
– Time
– Total chloride content of the lixiviant
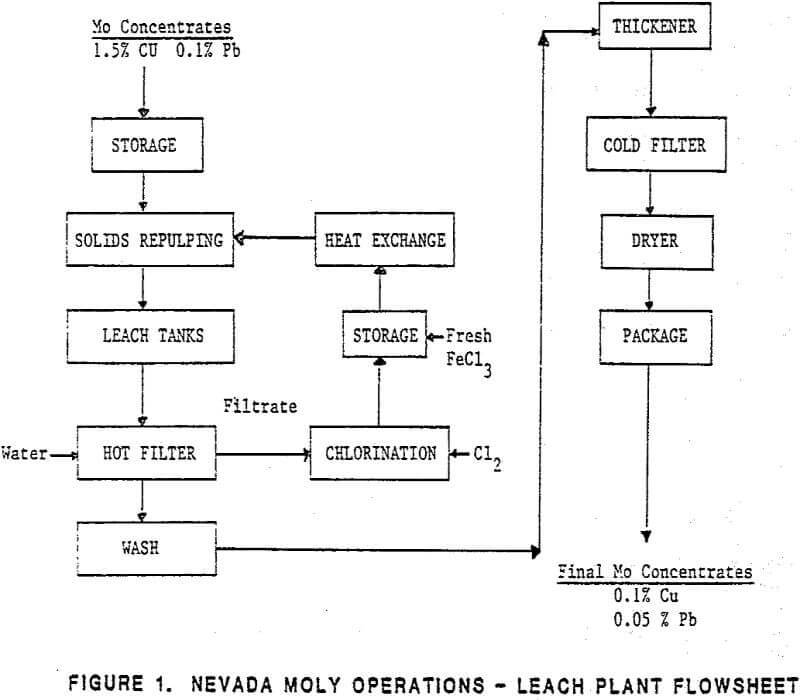
General Flow Sheet
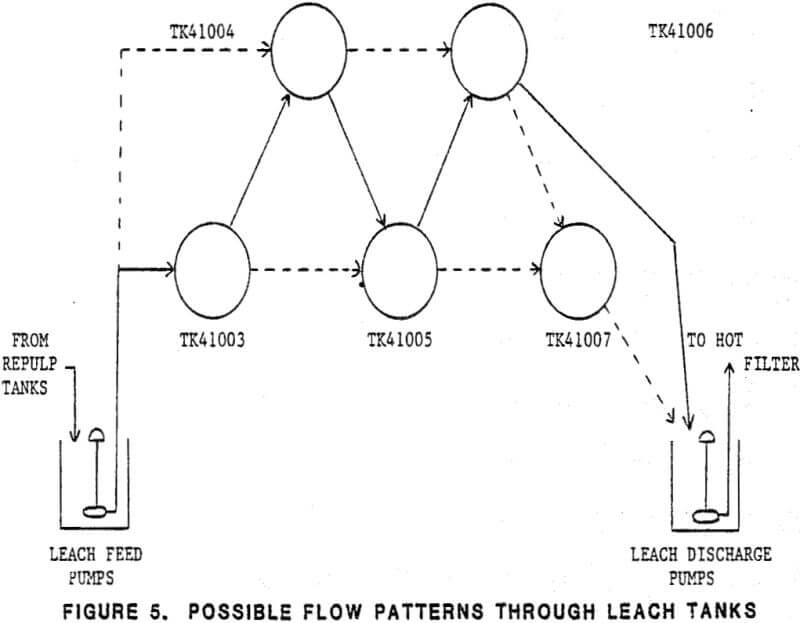
Molybdenum concentrates are transported from the Nevada Moly concentrator via bulk truck and are fed into one of four feed storage bins. Feed to the leach circuit is prepared in 24 hour batches in an unleached concentrate day bin. Feed rate is controlled from the day bin into a slurrying tank where hot ferric chloride solution is added, and the slurry reports to a series of four agitated leach tanks. The leached concentrate is separated from the hot ferric chloride leach solution using fiberglass drum filters, and is re-slurried with clean process wash water, thickened, and refiltered from the wash water.
The leach plant concentrate receiving, handling, and storage area consists of facilities for receiving concentrates from various delivery modes (bulk highway transport, barrels, or bulk shipping containers), storage of these concentrates, and batch blending of various concentrates prior to presentation as leach plant feed.
All concentrates are batch unloaded by gravity into a 20 tonne (22 ton) concentrate surge bin. To prevent dust losses during unloading, this bin is vented by a 1.42 m³/s (3,000 acfm) Mikropul dust collector equipped with polypropylene bags. Excellent dust control has been experienced with this system, with concentrate losses during unloading being minimal.
The concentrate leaching system consists of the unleached concentrate day bin, concentrate repulp circuit, and the agitated leach tank circuit. Here molybdenum concentrates are slurried with hot ferric chloride leach solution to dissolve the copper and lead contaminants present.
The primary function of the filtration and thickening circuits is to recover spent ferric chloride leach solution from the leached moly concentrates. Since approximately 2.5 percent of the ferric chloride in the leach solution reports with the filter cake during the first separation, the cake must be re-slurried with fresh water, thickened, and re-filtered. After the second filtration step, residual ferric chloride in the leached concentrate is less than 0.5 percent, and the risk of damage to downstream equipment is minimal.
Leached concentrates are advanced from the storage bin to the packaging plant as required. Final leached product is packaged in either 1360 kg (3,000 lb.) bulk containers or 208 L (55 gallon) drums. Dust control in this area is provided by a 1.23 m³/s (2600 acfm) Mikropul dust collector.
Solution Regeneration
Approximately 20 percent of the ferric chloride in the leach solution is reduced to ferrous chloride in the leaching process. Spent leach solution is routed to the solution regeneration system where the ferrous chloride is regenerated to ferric chloride by reaction with liquid chlorine, and recycle for processing additional unleached concentrates. Additionally, fresh ferric chloride solution is added to replenish that lost in the bleed stream.
Performance of the chlorine injection system and leach solution transfer pumps has been excellent. The initial chlorine neutralization system utilized a bubble tank filled with caustic solution which proved to be totally inadequate in neutralizing fugitive fumes. This system has been modified using plastic saddles in the bubble tank and the gases are vented to a caustic scrubber.
The leach plant was designed to process 2.2 t/h (2.42 STPH) of molybdenite concentrates averaging less than 1.5 percent copper. Startup in late August 1982 was at 1.0 t/h (1.1 STPH) and was gradually increased to 1.5 t/h (1.65 STPH) in November 1982. These tonnages were prior to finding the feed scale problems and therefore were thought to be higher than stated above at the time. The mechanical problems discussed earlier were very pronounced in early 1983 when the feed rate was raised to 2.2 t/h (2.42 STPH). The condition of the filters, scrubber, and screw conveyors required that the feed rate be reduced or extended periods of downtime occurred. After many of the mechanical problems were alleviated, production increased in April of 1983 to above 1.9 t/h (2.1 STPH) and plant availability in April was 89 percent.