Table of Contents
Domestically produced primary lead is won from lead sulfide concentrates by a smelting process consisting of sintering, blast furnace reduction with carbon and refining. Unfortunately, this pyrometallurgical practice creates gaseous sulfur oxides and particulate lead, which must be controlled to prevent air pollution. For some smelters, meeting environmental regulations requires production curtailment at times, and for some, the regulations threaten eventual closure. As a result, independent mine operators are experiencing difficulties in marketing their concentrates, and in some cases, are looking to chemical processing as an alternative to smelting. Smelter charges and transportation costs have also become limiting factors in the exploitation of newly discovered deposits. Because it generally is not economically feasible to build small lead smelters, the Federal Bureau of Mines has investigated hydrometallurgy to provide a simple, inexpensive, low-pollution process to treat lead concentrate on a small scale with a reasonable capital investment.
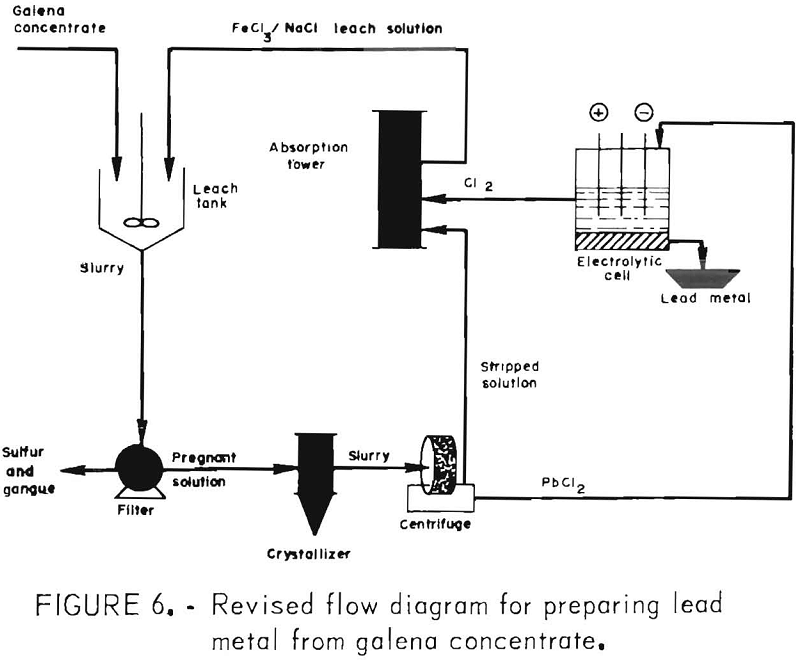
The objective of the present investigation was to optimize the method developed for treating galena concentrate that would produce lead by FeCl3 leaching and fused-salt electrolysis and would minimize sulfur oxide and lead emissions. Preliminary work on the project was reported in 1974. The concept, shown in figure 1, involves treating galena flotation concentrate with ferric chloride solution to obtain an impure lead chloride,
PbS + 2FeCl3 → PbCl2 + 2FeCl2 + S°……………………………………………………..(1)
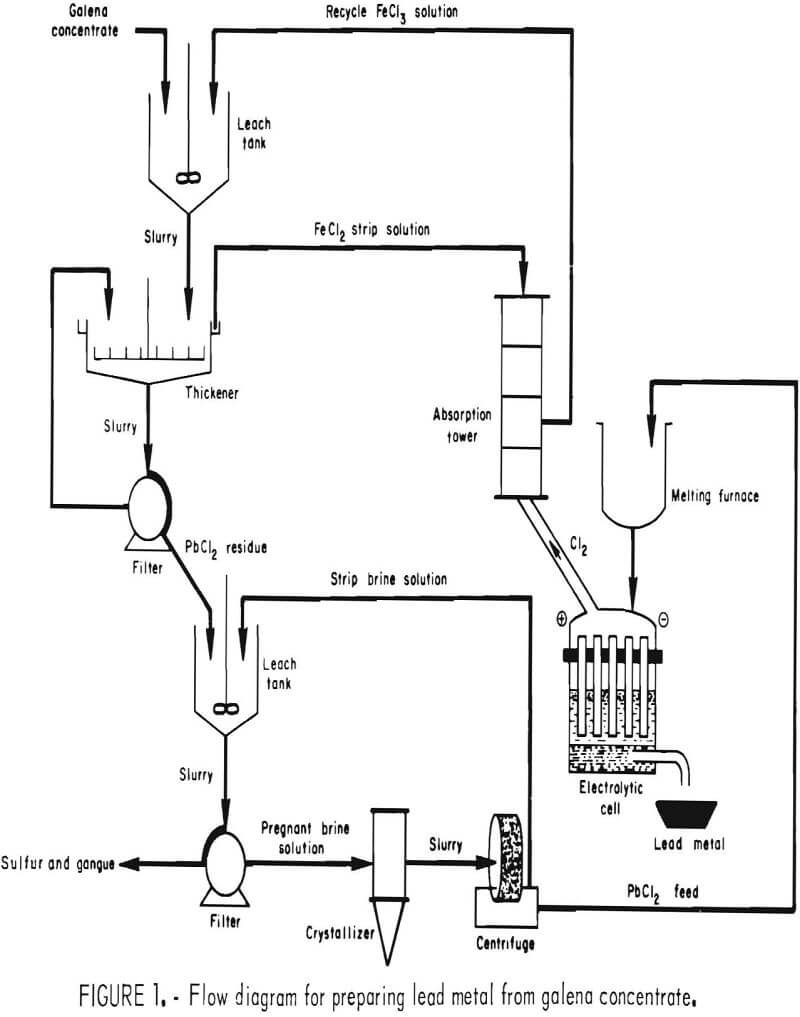
After filtration, the leach residue-composed of PbCl2, elemental sulfur, and gangue is agitated with hot brine (NaCl solution) to dissolve the PbCl2. The brine then is cooled to crystallize out pure PbCl2 , which can be electrolyzed in a fused-salt bath to obtain lead and chlorine gas. The chlorine is absorbed in spent leach solution to regenerate FeCl3 for further use. Other values (S°, Ag, Cu, Zn) may be recovered by additional treatment of either the leach residue or leach solution.
Although a ferric chloride leach has been used successfully in the treatment of chalcopyrite, nothing has been reported in this country concerning application of the technique to galena. However, considerable work has been done in other countries. The use of ferric sulfate (Fe2(SO4)3) for this purpose was described in an earlier Bureau of Mines publication. Leaching galena with Fe2(SO4)3 has an advantage in that insoluble PbSO4 is formed, making it possible to extract much of the impurity content while leaving the lead as an insoluble precipitate for subsequent treatment. The reaction rate of PbS with FeCl3 is much faster, however, Also, the leach product, PbCl2, lends itself to fused-salt electrolysis, which offers advantages such as production of high-purity metal in a molten form, a low energy requirement, and the possibility of employing very high current densities to minimize equipment size and capital costs. No attempt was made in the preliminary work to optimize leaching parameters. In the present investigation, the effect of operating variables was determined using lead concentrate from the Coeur d’Alene district of Idaho.
Effect of Operating Variables
All tests were made with flotation concentrate having the following analysis, in wt-pct:

The concentrate also contained 40.9 oz/ton silver and a trace of gold. Minerals identified by petrographic examination were galena, marmatite, pyrite, marcasite, and quartz. The silver was partly associated with tetrahedrite [(Cu, Fe)12Sb4S13] and partly with galena. Since Pb, Fe, Cu, Zn, Sb, Ag, and S were the main constituents, the extent of their extraction was determined in leach tests.
Particle Size
The first variable to be investigated was particle size. Concentrate was ground dry in a ball mill for various periods of time with the results shown in table 1. Galena is very soft (2.5 on Moh’s scale) and friable and is, therefore, easily reduced in size even with a 15-min grind. Samples of the concentrate, after grinding, were treated with a slight excess (110 pct theoretical) of FeCl3 solution; the amount of solution was based on the lead content (68.0 pct) of the concentrate. To prepare the leach solution, 1,600 g of FeCl3·6H2O were dissolved in enough water to make 2 liters. The dilution was based on the solubility of FeCl2·4H2O; in other words, if all of the Fe(III) were converted to Fe(II) during the leach, the resulting solution would be saturated with respect to FeCl2·4H2O.
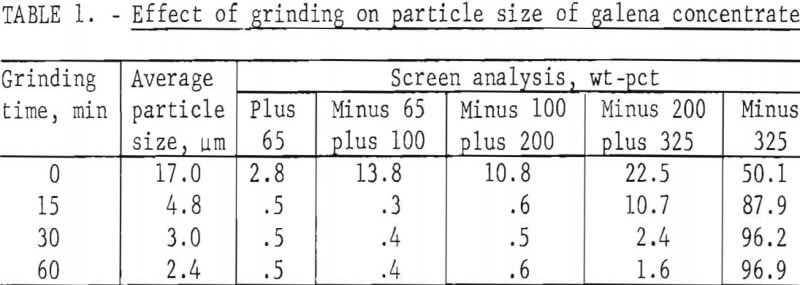
In the tests, 200 g of concentrate were agitated with 500 ml of FeCl3 solution at ambient temperature (20° to 25° C) for periods of 1, 2, 4, and 8 hr. After the specified times, the slurries were filtered, and the filter cake was added to enough hot brine (3 liters, 300 g NaCl/l, 100° C) to dissolve all of the PbCl3 present. The mixture was agitated for a few minutes and then filtered; the solids were washed with water, dried, and analyzed. Results are given in table 2.
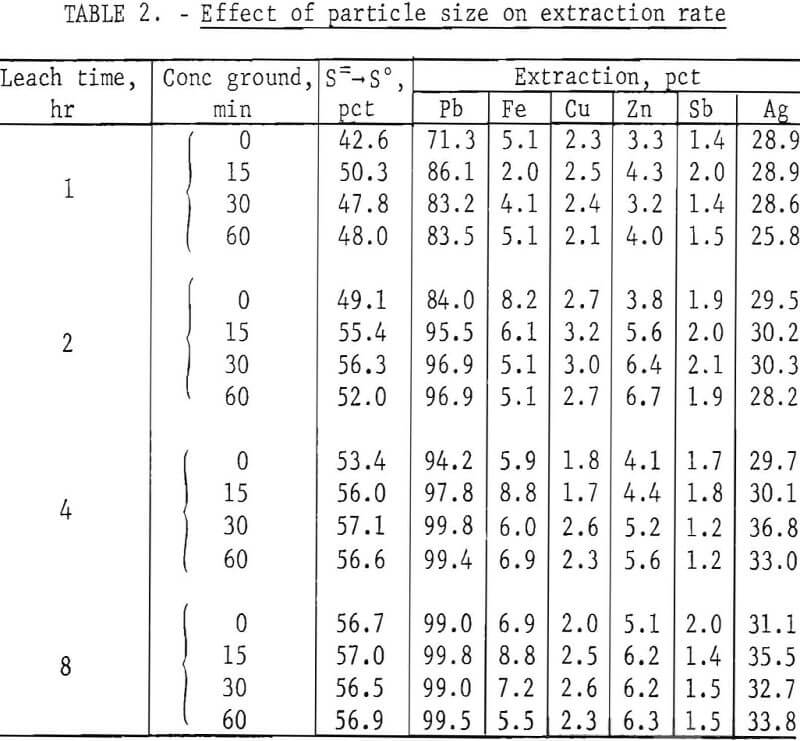
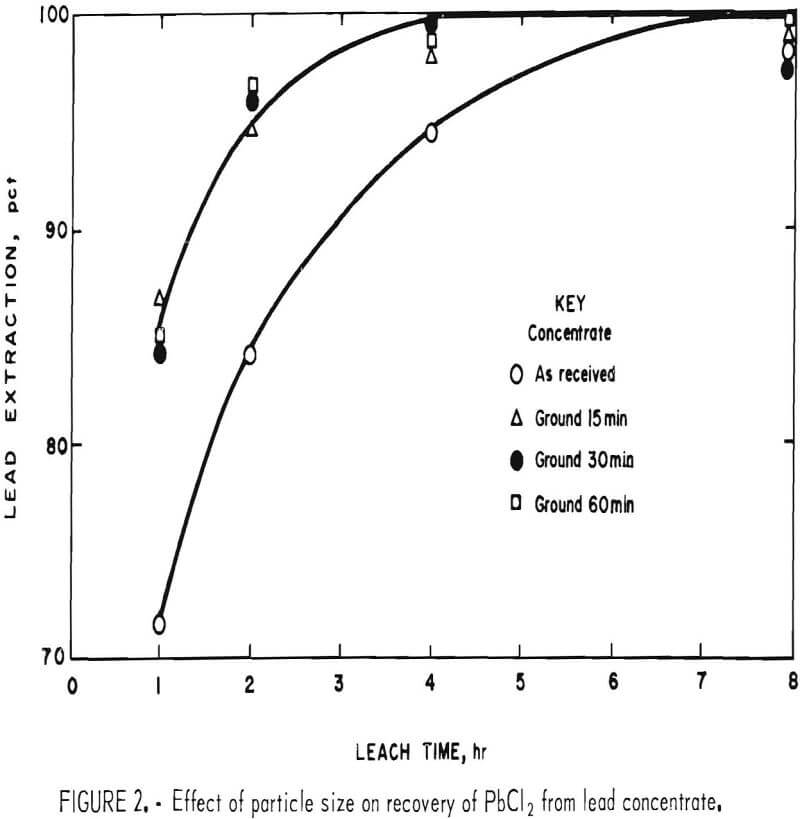
It is apparent from figure 2 that grinding the concentrate for 15 min was beneficial and that further size reduction had little effect. Extractions of components other than lead and silver were minimal. This is advantageous because chlorine combined with elements other than lead is not recovered in electrolysis and must be replaced.
FeCl3/PbS Weight Ratio
In the preceding tests, 200 g of concentrate were leached with 500 ml of FeCl3 solution containing 165 g Fe(III) per liter. This was about 10 pct more than the theoretical amount (445 ml) required to react with the PbS present. To determine the effect of different FeCl3/PbS weight ratios on the reaction rate, 200 g of concentrate ground for 15 min was agitated with 400, 600, 800, and 1,000 ml of the FeCl3 solution for periods of 1, 2, 4, and 8 hr at ambient temperature (20° to 25° C). Leach residues were treated with brine as in the previous tests, and the solids were washed with water, dried, and analyzed. Results are shown in table 3.
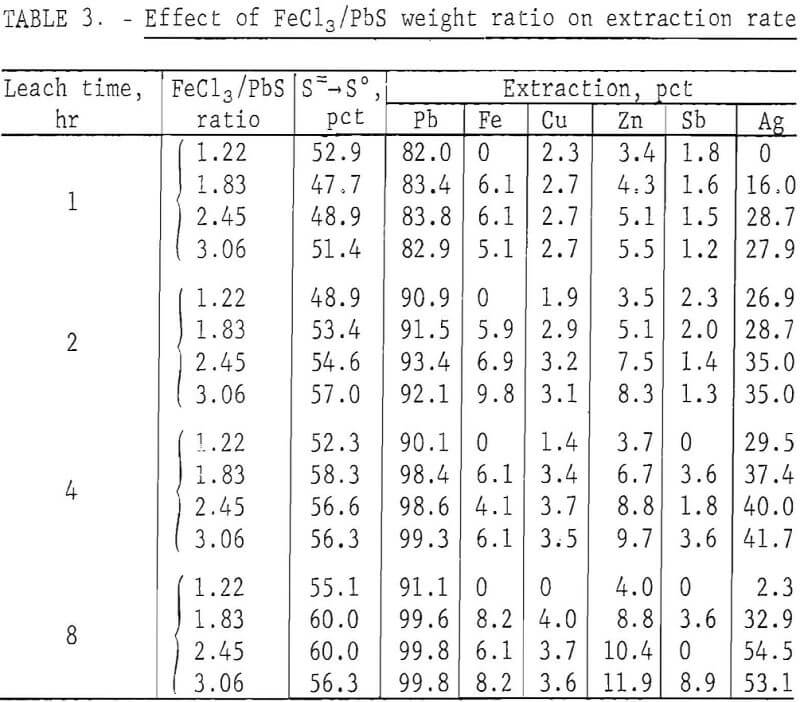
To satisfy equation 1, 1.36 parts FeCl3/part PbS would be required. Figure 3 shows that no advantage is gained by using more than this amount.
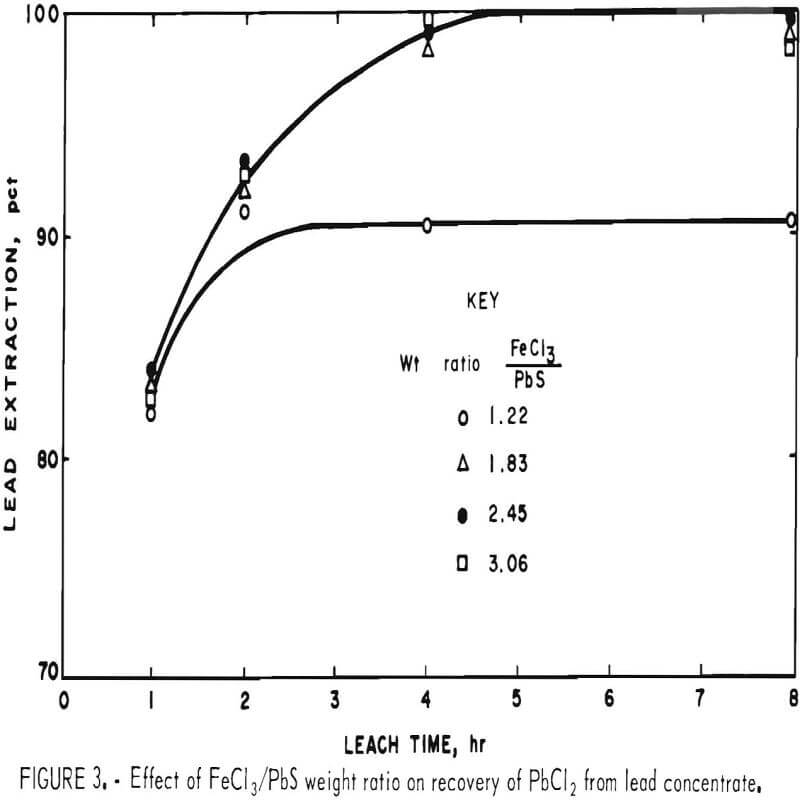
Increasing the ratio of FeCl3 to PbS had no effect on the reaction rate and had only a minor effect on the extraction of elements other than lead.
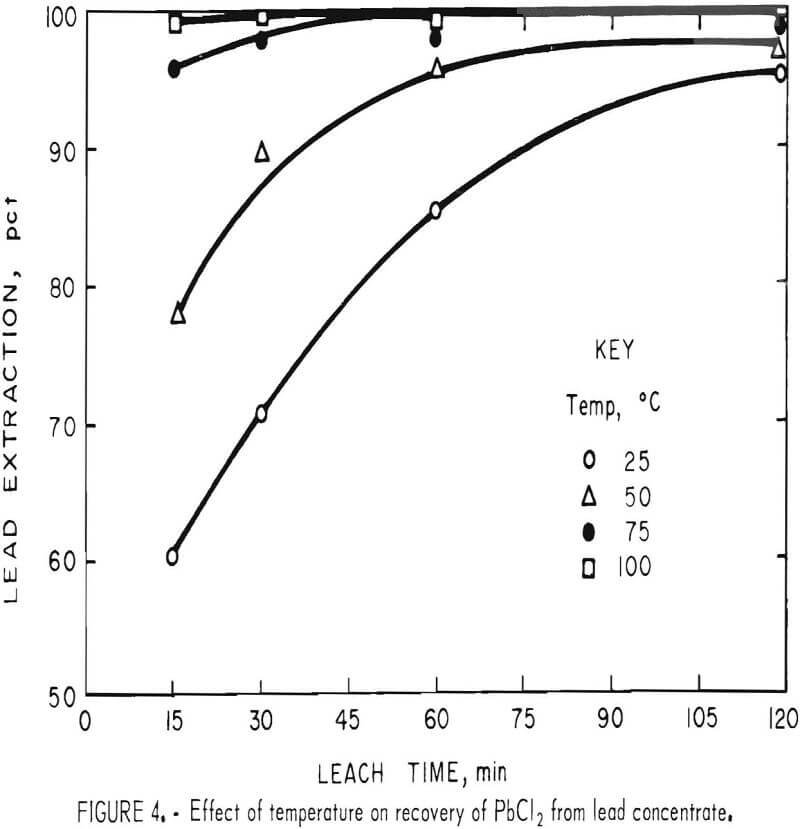
Temperature
To determine the effect of temperature, lead concentrate (200 g), ground for 15 min, was treated with a slight excess (500 ml) of FeCl3 solution for periods of 15, 30, 60, and 120 min at temperatures of 25°, 50°, 75°, and 100° C (±5° C). After extracting PbCl3 with brine, the leach residues were analyzed with the results shown in table 4.
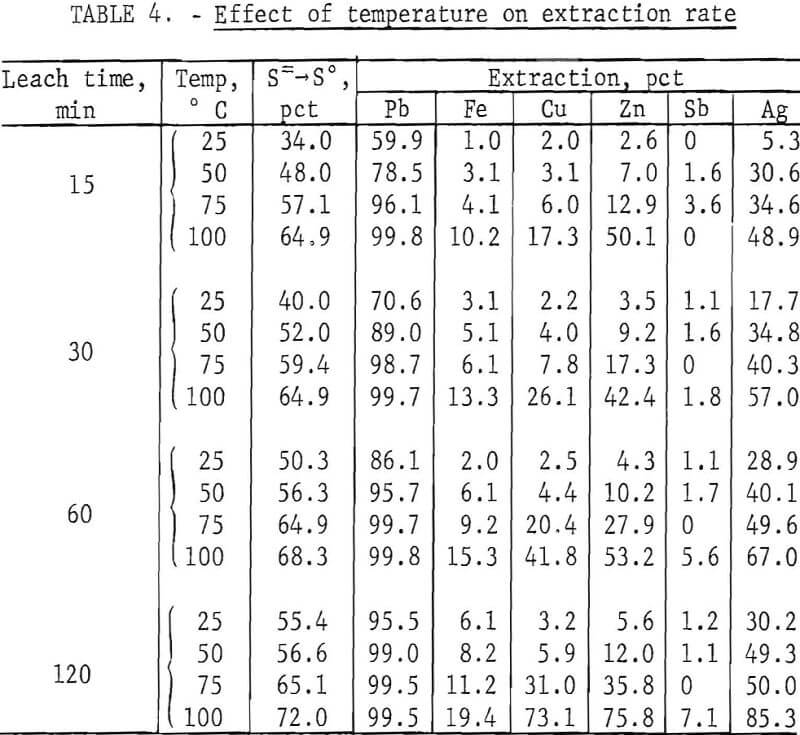
It is evident (fig. 4) that temperature had a very pronounced effect; a satisfactory lead recovery (99.8 pct) was obtained with only a 15-min leach at 100° C. Longer times resulted in the further dissolution of elements other than lead. As stated before, this is a disadvantage because the only chlorine recovered for use in the regeneration of Fe(III) is that associated with lead. The chlorine, in the form of FeCl3, which reacts with Fe, Cu, Zn, Sb, and other elements, must be replaced in order to maintain the same Fe(III) concentration in the leach solution. The shortest possible leach time is, therefore, desirable.
Heating not only increases the reaction rate but also greatly improves the settling and filtering characteristics of the leach residue. Any practical application, therefore, would probably require a hot leach. Since brine (NaCl solution) must be heated in the second step (fig. 1) to obtain pure PbCl2, an obvious deduction would be to combine the FeCl3 and NaCl solutions, thus eliminating one filtration step.
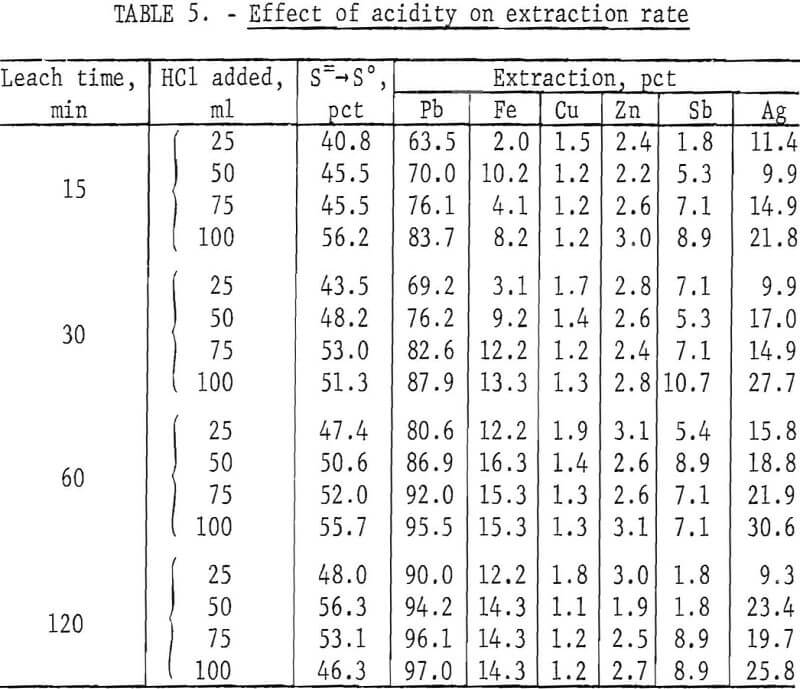
Acidity
The FeCl3 leach solution used in the previous tests was not acidified. However, lowering the pH might be expected to increase the reaction rate. To determine the effect of acidity, lead concentrate (200 g) was treated with 500 ml of solution containing a slight excess of ferric chloride (400 g FeCl3·6H2O) plus 25, 50, 75, and 100 ml of concentrated (38 pct) HCl. Tests were made at ambient temperature (20° to 25° C) for periods of 15, 30, 60, and 120 min. Heating was not used in this case to avoid loss of HCl. Results are shown in table 5.
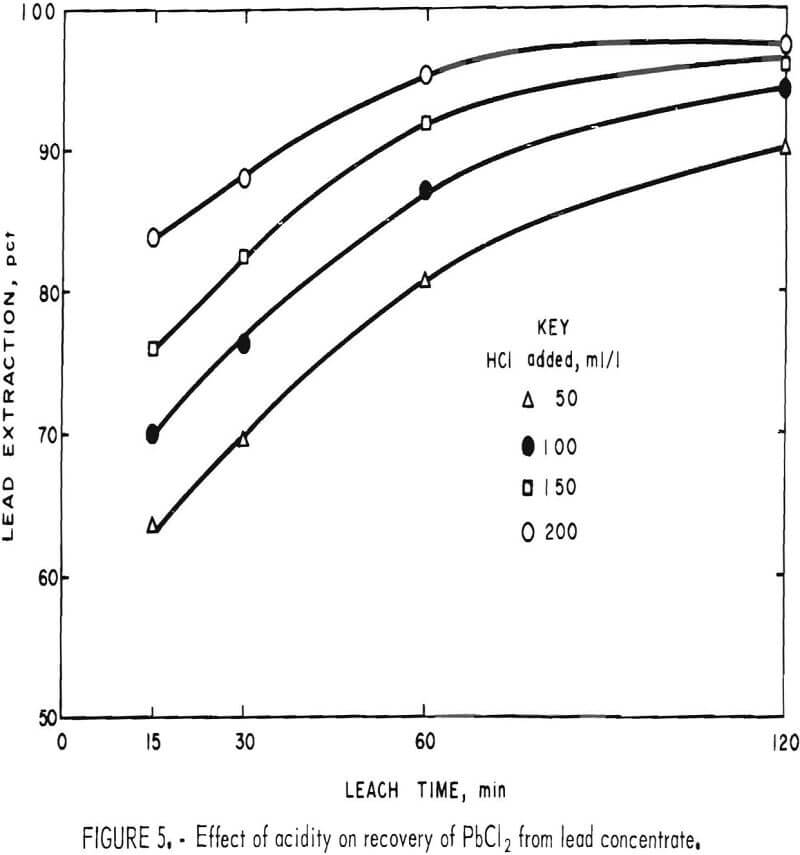
HCl reacts slowly with PbS to give PbCl2 and H2S. In the presence of FeCl3, however, H2S is oxidized to elemental sulfur and no gassing occurs. Figure 5 shows that acidity has a definite effect on the reaction rate, although not enough to compensate for the disadvantages of HCl such as cost, enhanced corrosion, and air contamination.
Proposed Process for Recovery of Metal Values from Galena Concentrate
The aforementioned tests indicated that, for optimum results, the concentrate should be ground to 90 pct minus 325 mesh and leached with the theoretical amount of FeCl3 at 100° C for 15 min. These conditions were employed in the following work, using a combined FeCl3/NaCl leach to simplify the process (fig. 6) and reduce the heat requirements.
FeCl3/NaCl Leach
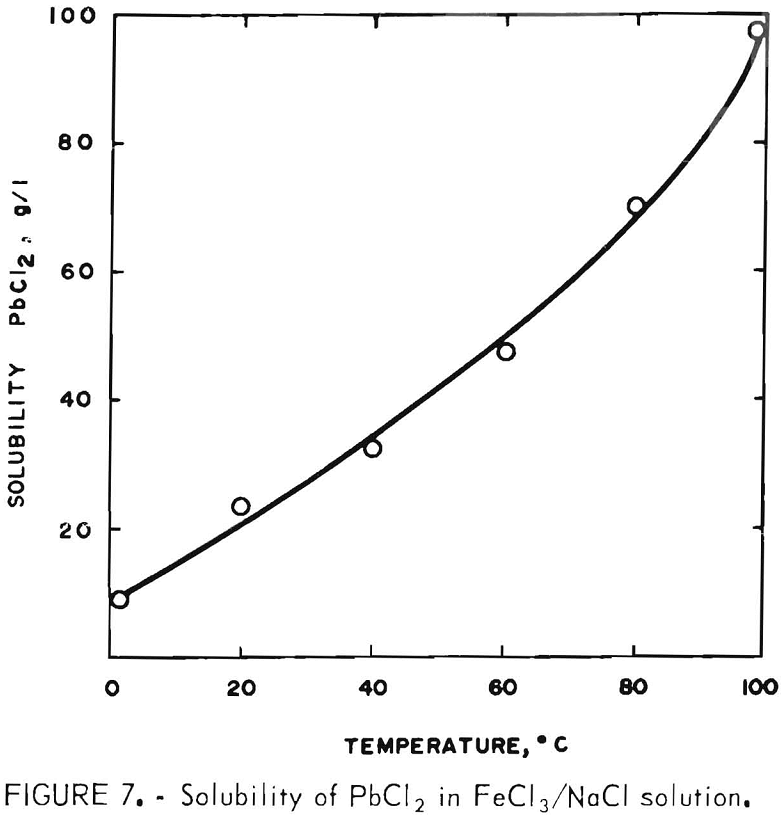
For this phase of the investigation, the leach solution was made up by adding 400 g FeCl3·6H2O to 2 liters of brine saturated with both NaCl and PbCl2. The FeCl3 addition displaced some of the NaCl, giving a filtrate containing 200 g FeCl3·6H2O, 230 g NaCl, and 24 g PbCl2 per liter (20° C). It was hoped that 2 liters would be sufficient to treat 200 g of concentrate. To determine exactly how much was required, the solubility of PbCl2 in the solution was determined at temperatures of 0°, 20°, 40°, 60°, 80°, and 100° C (±1° C) with the results shown in figure 7 (concentrate was used to reduce most of the Fe(III) to Fe(II) in order to duplicate conditions present in an actual leach).
Two hundred grams of concentrate will produce 182 g PbCl2 so it is apparent that, at 100° C, 2.5 liters of the FeCl3/NaCl
solution is needed to dissolve the PbCl2 formed in the reaction. This volume was used in all subsequent tests even though it represented a large excess (140 pct theoretical) of Fe(III). The latter should prove to be an advantage, however, in any practical application because it provides a cushion against variations in operating conditions. Exploratory tests with the FeCl3/NaCl combination showed it to be equal or superior to FeCl3 alone. The reaction rate was slightly greater, and the PbCl2 obtained by crystallization was at least as pure as that from straight brine (NaCl solution).
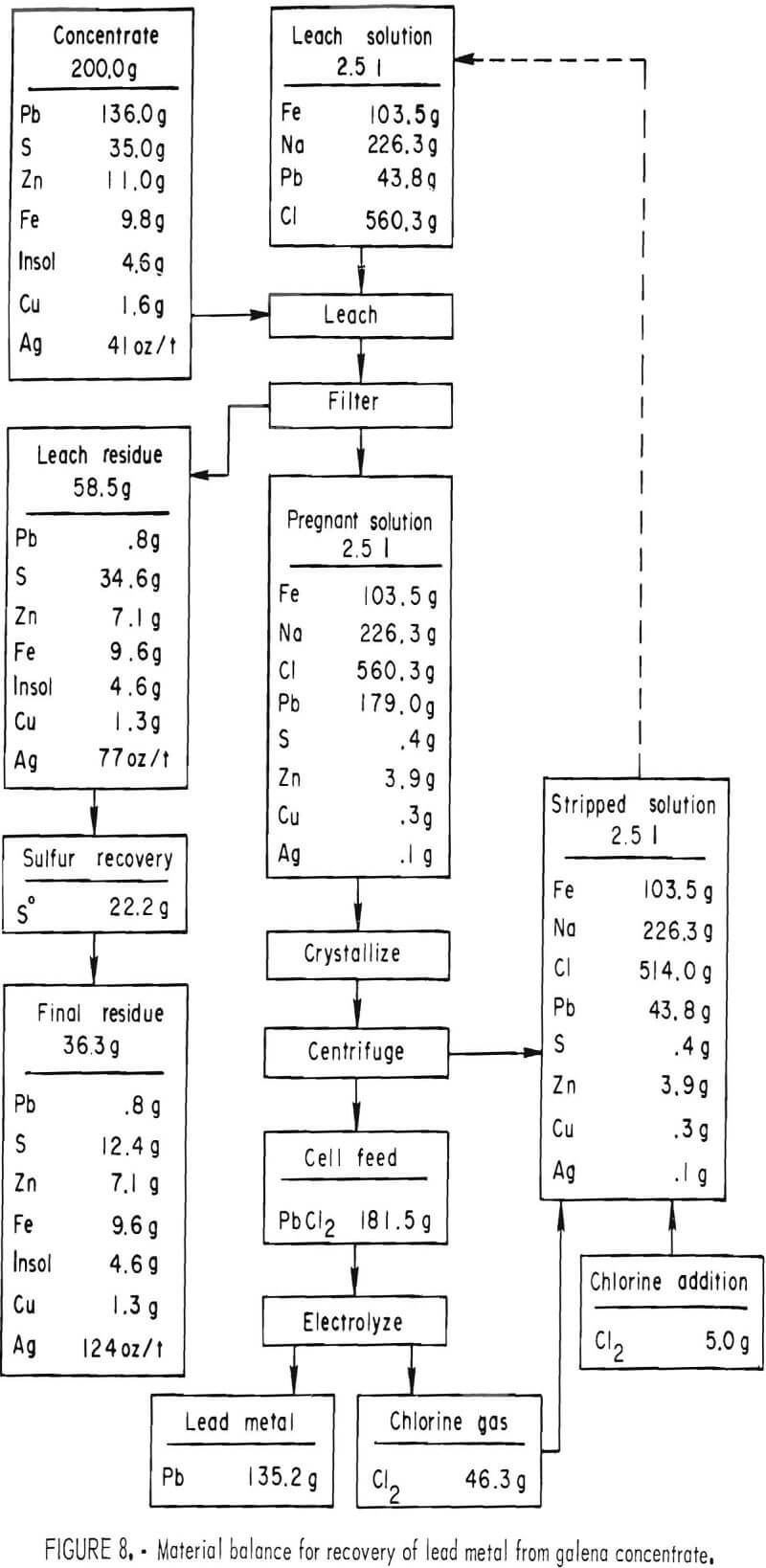
To determine the feasibility of recycle and the effect of impurity buildup, 200 g of concentrate, ground 15 min, were leached with 2.5 liters of the FeCl3/NaCl solution for 15 min at 100° C. After the 15-min period, the slurry was filtered hot, and the leach residue was washed, dried, and analyzed. The filtrate was cooled to room temperature to crystallize out PbCl3, and the volume was adjusted to 2.5 liters. After saturating the solution with chlorine, it was heated to 100° C and used to treat 200 more grams of concentrate. The aforementioned procedure was repeated 10 times. Analyses of the leach solution and leach residue after each step are shown in tables 6-7. Extractions obtained are given in table 8, and a material balance, based on a single leach, is shown in figure 8.
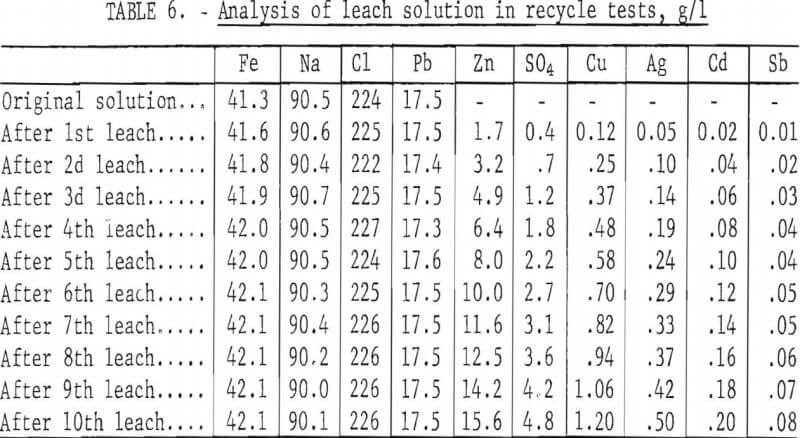
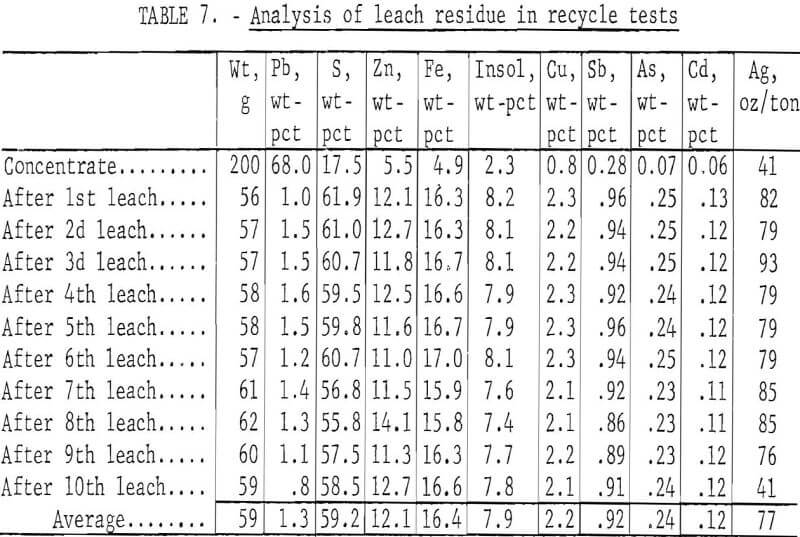
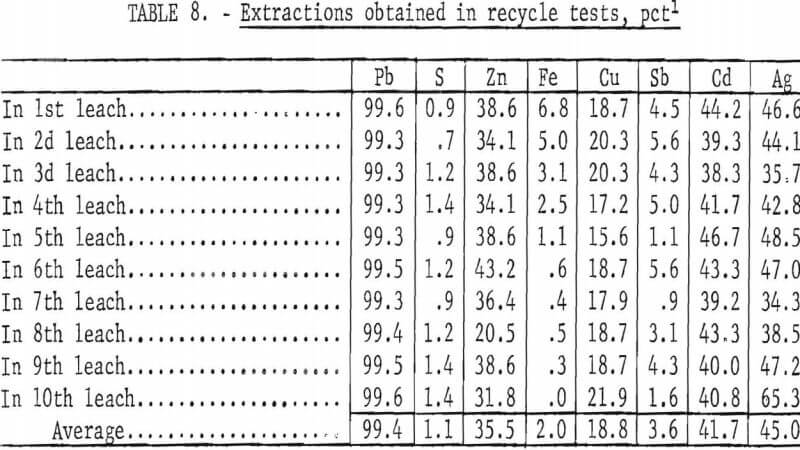
No difficulties were encountered in the tests; the effectiveness of the leach solution did not decrease with time, and impurity buildup was nominal. As expected, zinc proved to be the major impurity in the leach solution with sulfate, Cu, and Ag present in lesser amounts. Iron and antimony dissolved to only a limited extent. Arsenic and bismuth did not appear to be soluble; the arsenic may have formed insoluble ferric arsenate, and bismuth may have been converted to insoluble oxychloride.
No sulfate is formed in the reaction between PbS and FeCl3. However, a small amount of PbSO4 (anglesite) was present as an oxidation product on the surface of the mineral particles. The PbSO4 dissolved during the leach and had to be removed from solution; otherwise, it would eventually contaminate the PbCl2 product and interfere with electrolysis (sulfates are detrimental because they give SO2 and O2 at the anode, attacking the graphite. When the leach solution was heated prior to the addition of concentrate, a precipitate was observed to form which reported with the leach residue. This was found to be sodium jarosite [Na2Fe6(SO4)4(OH)12], a very insoluble, crystalline compound that develops in the presence of alkali ions at certain acidities, temperatures, and reaction times. The reaction,
6FeCl3 + 2NaCl + 4PbSO4 + 12H2O → Na2Fe6(SO4)4(OH)12 + 4PbCl2 + 12HCl……………………..(2)
is favored by a pH of 1.3 to 1.7 (pH FeCl3/NaCl solution = 2.2) and a temperature of 90° to 95° C. Acid produced must be neutralized, preferably with soda ash, to maintain the desired pH. Jarosite will remove both sulfate and excess iron from solution, but to be effective, a longer reaction time is required than that (15 min) used in the tests.
Altogether, 1,815 g of PbCl2 were produced. To determine what grade of metal could be obtained, the chloride was electrolyzed in a fused KCl/LiCl bath at 500° C, using a current density of 10 amp/sq in. This gave 1,352 g of lead at a current efficiency of 95 pct and an energy requirement of 0.30 kW·hr/lb. The purity of the metal is compared in table 9 with the ASTM specification for corroding-grade lead, the purest form sold commercially. The lead produced by electrolysis met ASTM specifications except for silver, which could be easily removed by the Parkes process.
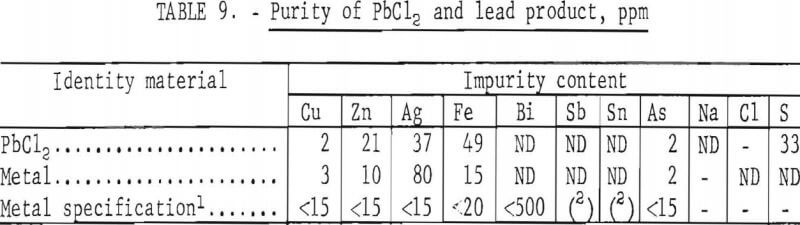
In the proposed process, the largest amount of energy is required to heat the FeCl3/NaCl solution. This is partially offset by the heat of reaction, necessitating a total heat input (100 pct thermal efficiency) of 1,825 Btu per pound of lead at a cost of $0.002 if natural gas ($1.20/Mcf) were used. Because of the limited solubility of PbCl2, large volumes of solution must also be handled, about 3,000 gallons or 400 cubic feet per ton of concentrate. Fortunately, the leach time is short (15 min) so equipment sizes are reasonable. The high current density possible in electrolysis also minimizes the size of equipment needed for this part of the operation.
Recovery of Byproducts
All of the aforementioned tests were concerned primarily with the recovery of lead because this is the chief value in most lead concentrates. Concentrate from the Missouri lead belt, where 85 pct of the lead in the United States originates, contains very little silver. Concentrate from the Coeur d’Alene district, however, contains a significant amount of silver, which must be extracted. Other constituents, such as copper and zinc, are not of great importance but would contribute to the economics of the process. Approximately 45 pct of the silver, 19 pct of the copper, and 36 pct of the zinc dissolved in the FeCl3/NaCl leach (table 8), giving a solution containing 0.5 g/l Ag, 1.2 g/l Cu, and 15.6 g/l Zn (table 6). These concentrations could, of course, go much higher before the limit of solubility was reached. It was found that Ag, Cu, and Sb could be reduced to <1 ppm by agitating the solution, heated to 90° C, with an excess (50 g) of granulated lead for 15 min. Any ferric iron present was reduced to ferrous at the same time. Recovering silver and copper in this way is advantageous because it converts the combined chlorine to PbCl2, making it available for regeneration of Fe(III). The silver could also be cemented out on copper before removal of the copper with lead. Copper and silver obtained as described may be made into salable products by conventional means.
Zinc chloride in concentrated chloride solutions forms an anion, (ZnCl4)²-, which can be adsorbed on anion exchange resins. Zinc can then be recovered subsequently by elution with water, leaving the resin in its original form. To determine if this were a feasible approach for separating zinc from the ferric chloride leach solution, 400 g of Dowex 1-X4 anion exchange resin was added to the solution after removal of Ag, Cu, and Sb. The slurry was stirred at ambient temperature for 30 min and then filtered. This reduced the zinc concentration from 15.6 to 0.3 g/l and also removed the small amount of cadmium present. No attempt was made to optimize further conditions involved in ion exchange treatment. The ZnCl2 obtained in this manner could be easily converted to ZnSO4, a salable product.
In the tests described, most of the iron and part of the sulfate entering the leach solution were precipitated as sodium jarosite. The Ag, Cu, and Sb were cemented out on metallic lead, and the Zn and Cd were adsorbed on an anion exchange resin. Only the remaining sulfate had to be removed to restore the leach solution to its original composition. It was determined that the sulfate concentration (4.8 g/l) could be lowered to 1.7 g/l by the addition of the theoretical amount of calcium choride, leaving the solution saturated with gypsum (CaSO4·2H2O). Considerable time was required for a precipitate to appear because CaSO4·2H2O tends to form a supersaturated solution even when seeded. In any case, jarosite formation appears to be by far the best method to control sulfate.
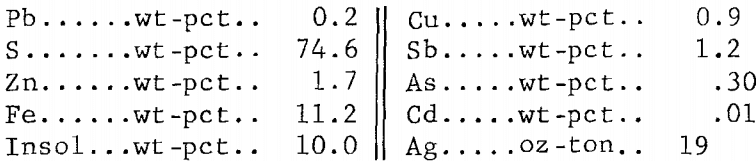
The 2,000 g of concentrate employed in the tests gave 585 g of residue containing more than half of the silver plus appreciable amounts of copper and zinc (table 7). Earlier work (table 4) had shown that these elements would dissolve in a ferric chloride leach if sufficient time were allowed. The leach residue was, therefore, agitated for 4 hr at 100° C with 4.0 liters of the FeCl3/NaCl solution; this amount provided enough Fe(III) to react with all of the Pb, Cu, Zn, and Ag present. The leach gave 464 g of residue having the following analysis:
The overall extraction of metal values was as follows in wt-pct·
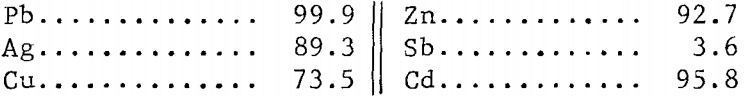
Metals dissolved in the leach mentioned could be recovered as outlined before; that is, lead by crystallization; copper, silver, and antimony by cementation; and zinc and cadmium by ion exchange. The solution after chlorinating could then be used for further treatment of either concentrate or leach residue.
The remaining solids contained 222 g (47.8 pct) of elemental sulfur. On a commercial scale, the residue could be discarded, burned to obtain sulfur dioxide for sulfuric acid production, or treated to recover the sulfur fraction. In the laboratory, the sulfur was extracted by adding the residue to 1 liter of perchloroethylene heated to 100° C. After filtration, very pure (99.95 pct S°) rhombic crystals were obtained on cooling. Perchloroethylene has a good solubility for sulfur, ranging from 25 g/l at 25° C to 422 g/l at 100° C. However, perchloroethylene probably would not be suitable for use on a large scale; ammonium sulfide appears to be a better solvent for a commercial application.
Conclusions
In conjunction with electrolysis, a ferric chloride-brine leach appears to have several advantages over smelting. It does not cause sulfur oxide and lead emissions, is suitable for a small-scale operation, is not capital intensive, requires little labor, and is not dependent on the use of scarce metallurgical coke. Over 99 pct of the lead in galena concentrate can be extracted in minutes. Energy requirements are reasonable although somewhat higher than that required for smelting. Valuable byproducts, such as Ag, Cu, and Zn, can be recovered from the leach solution by simple methods such as cementation and ion exchange, and the chief product, PbCl2, is pure enough to feed directly to an electrolytic cell to obtain corroding-grade metal. Chlorine given off in electrolysis is readily absorbed in spent leach solution, thus eliminating the need for chlorine handling and storage facilities. Because the reaction between FeCl2 and Cl2 is exothermic, a considerable amount of heat is also obtained in this way. The method not only avoids the generation of SO2 but yields elemental sulfur as a valuable byproduct. Apparent disadvantages include the use of a corrosive liquor, the need for makeup chlorine, and the handling and heating of large volumes of solution.

