Table of Contents
Results of this study demonstrate that multiple-oxide minerals can be processed to usable compounds by the techniques described in this paper.
The chlorination procedure offers an efficient method for extracting tantalum, columbium, uranium, titanium, thorium, and the rare-earth elements from their ores. Separation of the chloride products into four groups is of additional benefit for processing materials such as euxenite.
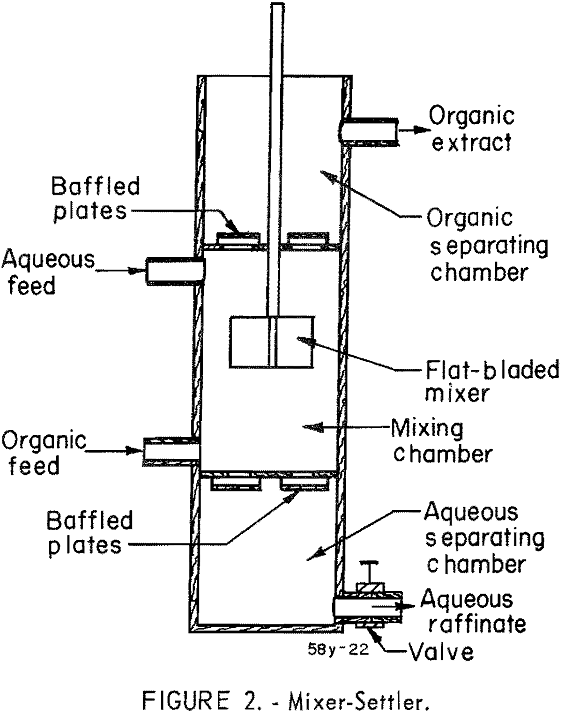
The solvent-extraction system used for continuous separation of tantalum and columbium yields pure compounds of the two metals. The mixer-settler units are simple in design and inexpensive to fabricate.
Conditions used for the hydrometallurgical processing of the residue from the chlorination and of the chloride eutectic were determined in laboratory-scale tests and then applied to the large-scale run. However, the extent of the small-scale tests was limited by availability of funds and time for the investigation. Recovery and purity of the products of the large-scale run were not as satisfactory as small-scale tests had indicated would be possible. However, the general procedures were adequate for the processing, and probably the recovery and purity of products would be improved considerably under optimum conditions for large-scale processing.
The overall results of processing the euxenite concentrate were satisfactory. During chlorination 95.9 percent of the euxenite was converted to chlorides. The solvent-extraction separation produced high-purity columbium and tantalum oxides. Approximately 80 percent of the tantalum and 86 percent of the columbium were recovered as pure compounds after passing through the separation system once. The rare-earth compounds in the chlorination residue were treated by hydrometallurgical procedures, which recovered 84 percent of the rare-earth elements in a product that contained 59 percent yttrium oxide. A 99 percent uranium oxide product was obtained by processing the eutectic product from the chlorination.
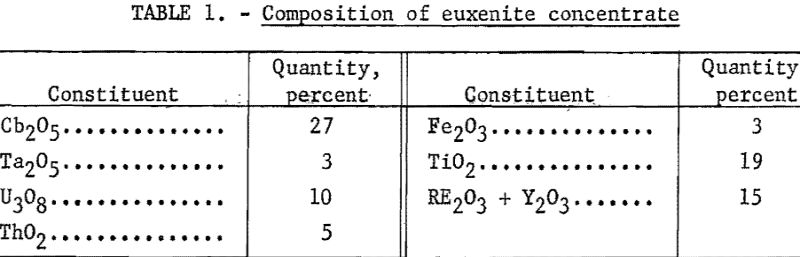
Euxenite now is the major domestic source of tantalum and columbium. The mineral is a complex, multiple oxide and, in addition to tantalum and columbium, contains substantial quantities of uranium, thorium, titanium, yttrium, and the rare-earth,elements. The company mill near Lawman, Idaho, has been described. One of the products is a euxenite concentrate with a typical composition as shown in table 1.
The objectives of the work were to process the euxenite and recover the following products: (1) High-purity columbium oxide, (2) high-purity tantalum oxide, (3) titanium tetrachloride, (4) a rare-earth product enriched in yttrium, and (5) uranium oxide.
The procedures used for extracting and purifying these compounds had been developed in earlier small-scale investigations. The valuable metals were chlorinated and separated into four groups: (1) Chlorination residue, containing rare-earth and thorium chlorides; (2) a sodium-iron-uranium chloride eutectic; (3) tantalum-columbium chlorides; and (4) liquid titanium tetrachloride. The tantalum-columbium chloride was processed further by solvent extraction to separate the two metals. The salt eutectic was treated to purify the uranium, and the chlorination residue was processed to purify the rare-earth elements. No further work was performed on the titanium tetrachloride product.
The presence of uranium, thorium, and possibly other radioactive materials in the euxenite necessitated compliance with AEC regulations 10 CFR, part 20, Standards for Protection Against Radiation. Precautions included approved filters in the ventilating system, air-line respirators and protective clothing for operating personnel, an areal monitoring system, and an air-sampling routine procedure. Data obtained from these precautionary measures indicated that the radioactive materials in the concentrate when properly handled should not present a health problem.
Experimental Procedures and Results
Extraction by Chlorination
Chlorination techniques have been used successfully to extract refractory metal compounds from multiple-oxide minerals, such as euxenite, on a small scale. Earlier work at this laboratory indicated that sodium chloride added to the chlorination charge prevents volatilization of the chlorides of iron and uranium. Nieberlein has reported removing iron and uranium chlorides from the volatile chloride stream by using a rock-salt column. In this test, a rock salt column was used because the removal of a major part of the iron and uranium from the chlorination residue would simplify later procedures needed to purify the rare-earth compounds.
Equipment
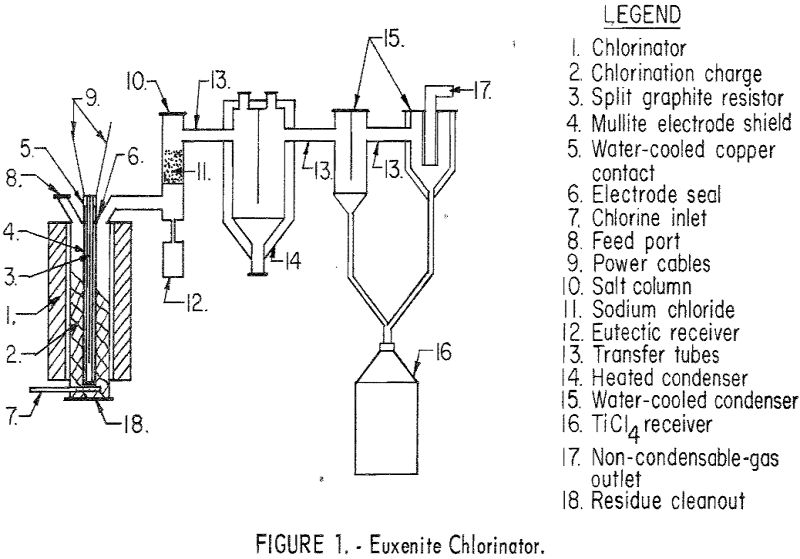
The chlorination apparatus for these tests consisted of four essential parts: A vertical-shaft chlorinator, a heated rock-salt column, a heated condenser, and a water-cooled condenser. The euxenite was reacted with chlorine in the vertical-shaft chlorinator, and the volatile chlorides passed from the reaction zone into a heated rock-salt column where the iron and uranium chlorides were complexed and separated from the gases as a low-melting eutectic. The tantalum, columbium, and titanium chlorides passed from the salt column into a heated condenser where the tantalum and columbium chlorides were removed from the volatile chloride stream. Gases from the heated condenser passed into a water-cooled condenser where the titanium tetrachloride was removed.
The chlorinator, 12 inches in diameter by 36 inches in height, was heated internally by a split graphite resistor. The walls of the reaction zone were formed by a 12-inch ID graphite tube. All metallic parts exposed to chlorine were fabricated of nickel. Figure 1 illustrates this apparatus in detail.
Procedure and Results
The chlorination charge of finely divided euxenite, granular carbon black, and sugar was dry-mixed 20:6:1 by weight in a Hobart dough mixer. After the materials were mixed intimately, water was added to the mixture until it had the consistency of a thick paste, which was formed into rods with a commercial extrusion device. The rods were ½ inch in diameter by about 1 inch in length. The extrusions were dried in a vacuum at 60° C. for 42 hours to remove the excess moisture. The dried rods were coked in an atmosphere of helium at 800° C, for 8 hours to decompose the sugar and then stored in a sealed container until used.
In a 24-hour chlorination run made to test the procedure and equipment, the equipment operated satisfactorily, except that chlorine leaked from the reactor. Because the extrusions for this run had not been coked, they disintegrated when they were heated in the chlorinator, so restricting the flow of gas through the reaction bed that chlorine was forced out of the reactor through the gaskets.
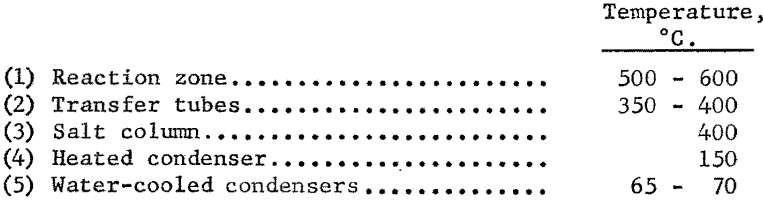
The second run was made to extract the valuable metals from 200 pounds of euxenite concentrate. During the first warmup, the reaction zone was filled with resistor carbon, and a stream of helium was passed slowly through the system. When all parts of the chlorinating apparatus had reached the desired temperatures, part of the resistor carbon was withdrawn, extrusions were fed into the reaction zone, and the chlorine was admitted. This run continued for 131 hours. The remainder of the resistor carbon was removed from the reaction zone after 25 hours of operation. After 59 hours part of the chlorination residue was removed to make room for additional briquets, and the run was continued until all the prepared charge material had been added. The following temperatures were maintained in the various parts of the chlorinating system:
The data for this run are shown in tables 2 and 3.
Four products of the chlorination were collected: (1) Nonvolatile residue, (2) nonvolatile eutectic of sodium-uranium-ferric chloride, (3) tantalum-columbium chloride, and (4) titanium tetrachloride. The nonvolatile residue contained rare-earth and thorium chlorides and represented complete recovery of these metals, as determined from the rare-earth and thorium content of the water-soluble part of the residue. The low-melting, nonvolatile complex of sodium-uranium-ferric chloride contained 66 percent of the uranium and the major part of the iron. The rest of the uranium remained with the chlorination residue. The tantalum-columbium chloride product represented a recovery of 96 percent of these metals. Recovered titanium tetrachloride represented 60 percent of the titanium present in the euxenite. Overall efficiency of extraction by chlorination was 95.9 percent; this value was calculated from the quantity of unreacted ore remaining in the chlorination residue. The chlorine efficiency for this test (Cl2 needed/Cl2 used x 100) was 70 percent.
Although chlorination of titanium was complete, a significant quantity of titanium tetrachloride was lost through the exhuast system to the atmosphere, because the water-cooled condensers available were not large enough to condense all of the titanium tetrachloride vapor. The additional cost of fabricating adequate condensers was not warranted, because this problem of condensing titanium tetrachloride has been solved by other investigators.
The results of the run demonstrated that the design of the chlorinating apparatus and that the procedures used were well suited for extracting and separating the metals in euxenite concentrate. All parts of the apparatus functioned satisfactorily except the heat source, which deteriorated during operation. The electrode shield failed after 59 hours of operation, and it was necessary to seal the opening between the shield and the electrode with asbestos packing to prevent leakage of the chlorine. This malfunction had no deleterious effect on the efficiency of the run. Shortly after the last of the charge material had been added, the split graphite resistor failed, a new one could not be inserted because chlorination residue fell into the space occupied by the broken electrode after it had been removed. When the furnace was dismantled, it was found that the mullite shield and much of the graphite liner had been attacked. A silica brick lining probably would be better than graphite.
Separation of Tantalum and Columbium
The second step in processing euxenite concentrate was separation of tantalum and columbium by solvent-extraction, using hydrofluoric acid-sulfuric acid-methyl isobutyl ketone, as reported by Tews and May. This system is based on the principles of coextraction of both tantalum and columbium from the aqueous feed solution into the organic phase to separate them from metallic impurities and preferential back extraction of the metals from the organic phase to separate tantalum from columbium. It can be applied to various ores by modifying the conditions. In this operation, tantalum and columbium were extracted from a hydrofluoric-sulfuric acid feed solution with methyl isobutyl ketone. The metal-laden solvent from this first coextraction then was contacted with a dilute hydrofluoric-sulfuric acid solution. This back extraction effected an incomplete separation of tantalum from columbium; most of the columbium was extracted by the aqueous phase; and most of the tantalum remained in the organic phase. The aqueous solution from the back extraction step was re-extracted with a small quantity of solvent to yield a pure columbium product. Re-extracting the organic phase from the back extraction two times with dilute sulfuric acid, resulted in a pure tantalum product. This product was stripped from the solvent with a 5 percent ammonium fluoride solution.
Equipment
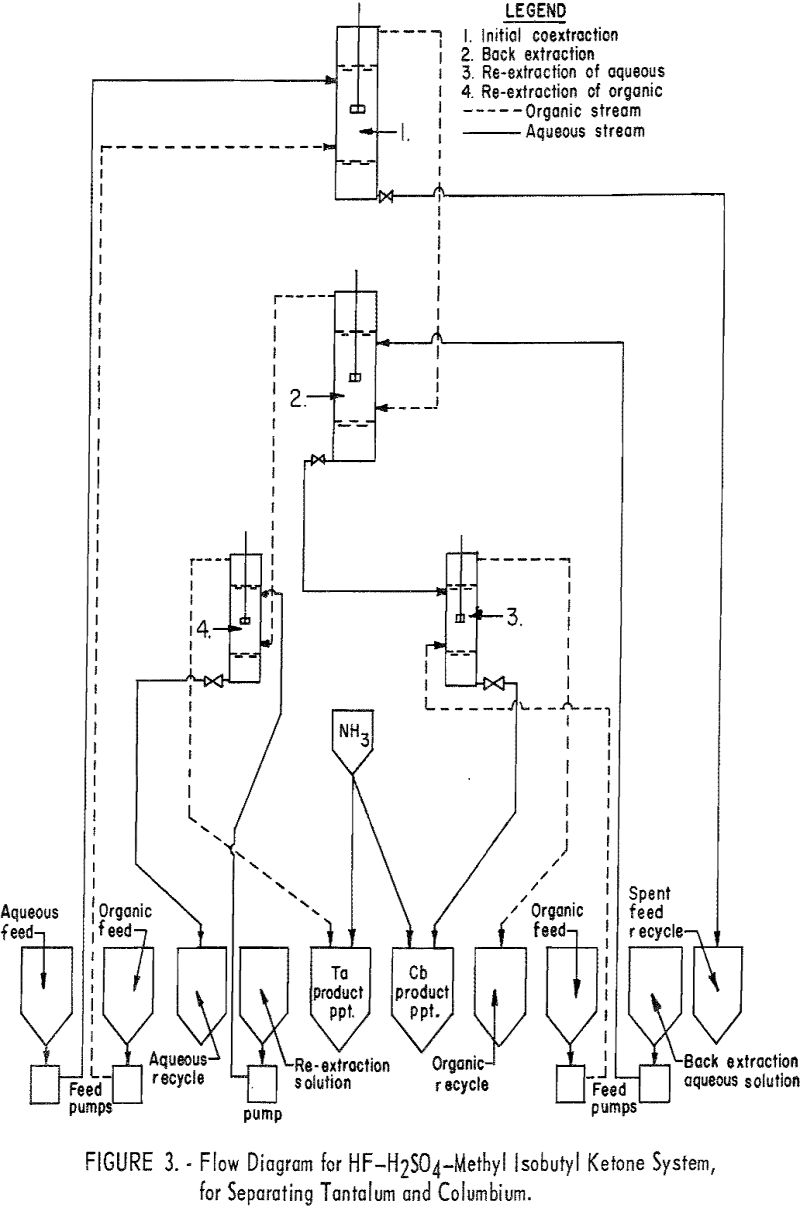
The equipment consisted of four mixer-settlers that were fabricated of polyethylene and were capable of processing approximately 3 to 4 pounds of mixed columbium and tantalum oxides per hour. Solutions were transferred from feed tanks to the mixer-settlers by acid-resistant proportioning pumps. The organic and aqueous streams from the mixer-settler units were transferred by gravity. The design of mixer-settler units (fig. 2) and the arrangement of contactors and pumps (flow diagram, fig. 3) are shown.
Procedure and Results
Two runs were made with mixed tantalum and columbium oxides that had been extracted from a euxenite concentrate. The first batch of hydrated oxides had been extracted from a euxenite concentrate at Mallinckrodt Chemical Works by a hydrometallurgical process. A sample of this material was ignited at 850° C. The moisture content was determined to be 15.3 percent, and the product contained 83 percent columbium oxide and 12 percent tantalum oxide.
After the feed solution was prepared by dissolving 50 pounds of hydrated oxides in 50 percent hydrofluoric acid, sulfuric acid and water were added to obtain the desired metal and acid concentrations. This feed solution was 5.6-normal in hydrofluoric acid, 9-normal in sulfuric acid, and contained 82.25 grams of mixed oxides per liter. Methyl isobutyl ketone was used as received.
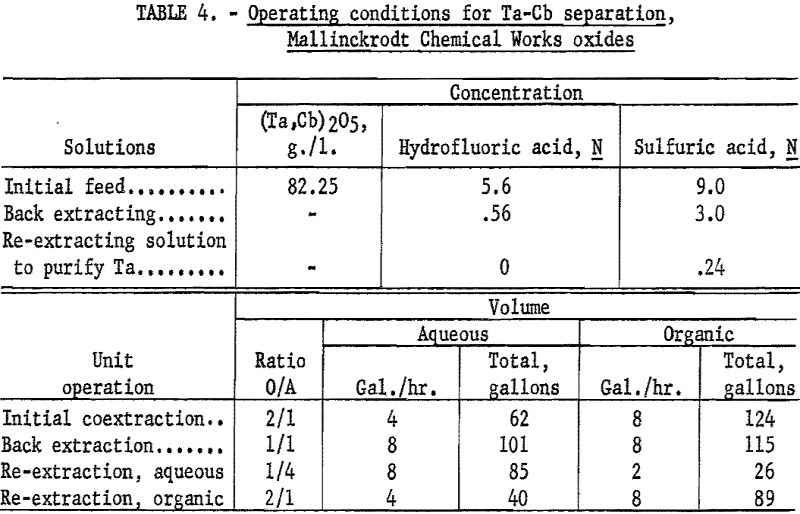
For the initial coextraction operation of the continuous run, aqueous feed solution and fresh solvent, at an organic-to-aqueous volume ratio of 2:1, were pumped into the uppermost mixer-settler of a four-unit assembly. After mixing, the phases disengaged and the metal-laden solvent flowed into the second mixer-settler while the spent feed solution was removed from the system. In the second or back extracting mixer-settler the solvent was contacted with a dilute hydrofluoric-sulfuric acid solution at a volume ratio of 1:1. The aqueous phase from the back extraction flowed to a third mixer-settler where it was contacted with fresh solvent in an organic-to-aqueous volume ratio of 1:4. This re-extraction removed tantalum impurity, and the aqueous stream from this unit contained the final columbium product. The organic phase from the back extraction flowed into the fourth mixer-settler and was contacted with dilute sulfuric acid in an organic-to-aqueous volume ratio of 2:1. The solvent stream from this phase, containing the tantalum product contaminated with 1 percent columbium, was necessarily contacted a second time with dilute sulfuric acid in an organic-to-aqueous volume ratio of 2:1 to obtain a pure tantalum product. Samples were taken periodically during the run of the spent feed solution from the initial coextraction and of the columbium product and tantalum product streams. When the run was completed, samples of the composite solutions were collected. Metal concentrations of the solutions were determined by ammonium hydroxide precipitation; composition of the resulting oxides was determined by spectrographic analyses. Operating conditions for this run are given (table 4).
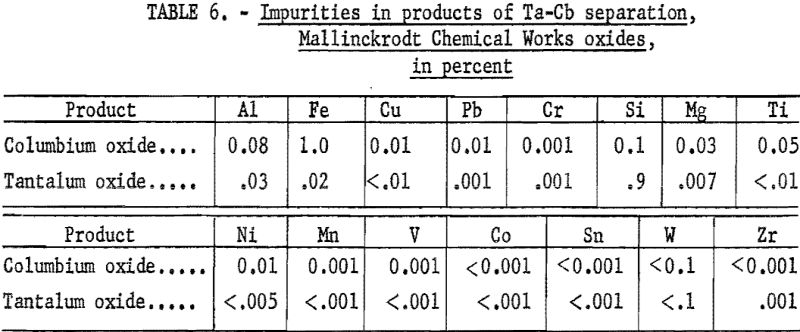
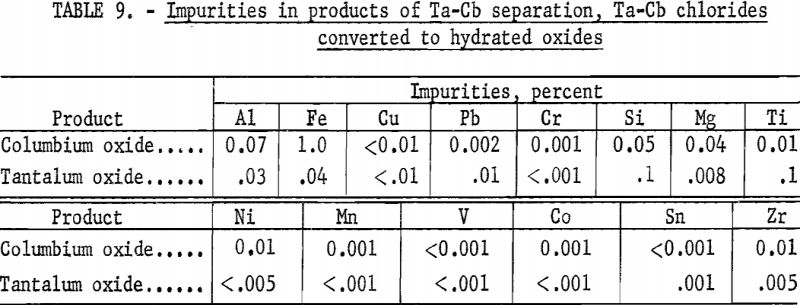
Table 5 shows the weights and tantalum-columbium composition of the intermediate and final products from this run. Table 6 shows the metallic impurities in the final products. Some impurities were introduced from Technical-grade hydrofluoric acid. The 70 percent hydrofluoric acid used in the aqueous solutions, except the ammonium fluoride stripping solution, contained a total of 3.14 grams of metal per liter, of which 1.9 gram per liter was iron.
The columbium product was precipitated from the aqueous solution with ammonium hydroxide and dewatered by means of a Moore leaf filter. The tantalum product was stripped from the solvent phase with a 5-percent solution of ammonium fluoride. Ammonium hydroxide was added to the stripping solution and the precipitated tantalum product was collected on a filter.
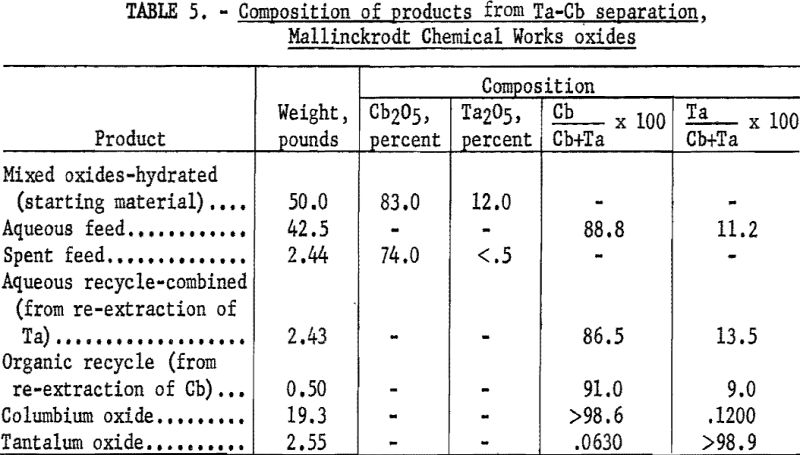
Recovery of 79.5 percent of the columbium and 86.6 percent of the tantalum was achieved. These values were calculated by dividing the weight of the final product by the weight of the columbium or tantalum oxide that was removed from the separation system during the run. The quantities of oxides that remained in the mixer-settlers at the completion of the run were not included with the weight of tantalum or columbium oxide that was used as the divisor in the recovery calculations. It must be emphasized that this operation was a short-term run; the length of the run was limited by the volume of feed solution. When all the feed solution was added, the run was stopped, and significant volumes of metal-bearing solutions were left in the mixer-settlers. None of the recycle solutions was returned to the system. The flow diagram (fig. 3) shows that the recycle solutions are the spent feed solution from the initial coextraction, the organic product from re-extraction of the aqueous phase, and the aqueous product from re-extraction of the organic phase. It has been calculated that if these solutions were recycled through the separation system at the efficiency calculated for the short term run, the recovery for a long term run would be 96 percent for columbium and 98 percent for tantalum.
Starting material for the second separation run was the tantalum and columbium chloride product from the euxenite-chlorinating run. Before the run the chlorides were hydrolyzed by adding them to water. The resulting hydrated oxides were washed with water until free of chloride ion, filtered, and dried. Because of inadequate control of the drying oven, the temperature rose to about 400° C. Experience has shown that hydrated oxides of tantalum and columbium dried at this temperature are difficult to dissolve in concentrated hydrofluoric acid.
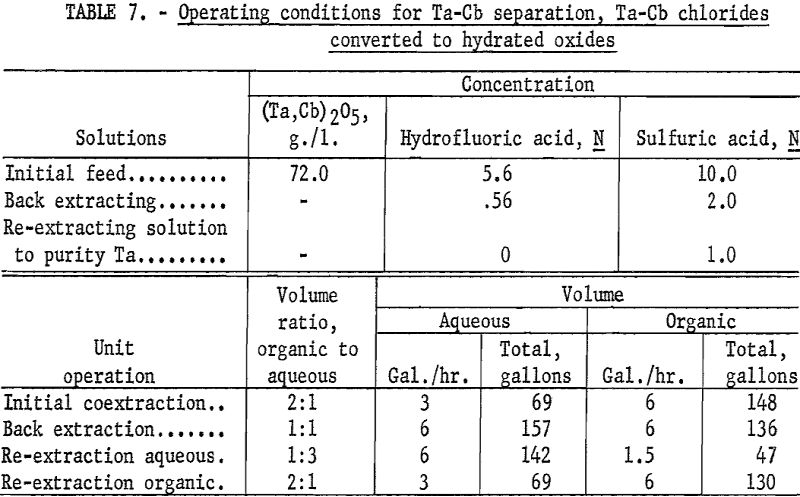
The feed solution was prepared by adding 60 pounds of the hydrated oxides to 70 percent hydrofluoric acid. Sulfuric acid and water were added to obtain the desired acid and metal concentrations. Approximately 12 pounds of the hydrated oxides did not dissolve and were removed by filtering. The final solution was 5.6 normal in hydrofluoric acid, 10 normal in sulfuric acid, and contained 72.0 grams of mixed oxides per liter.
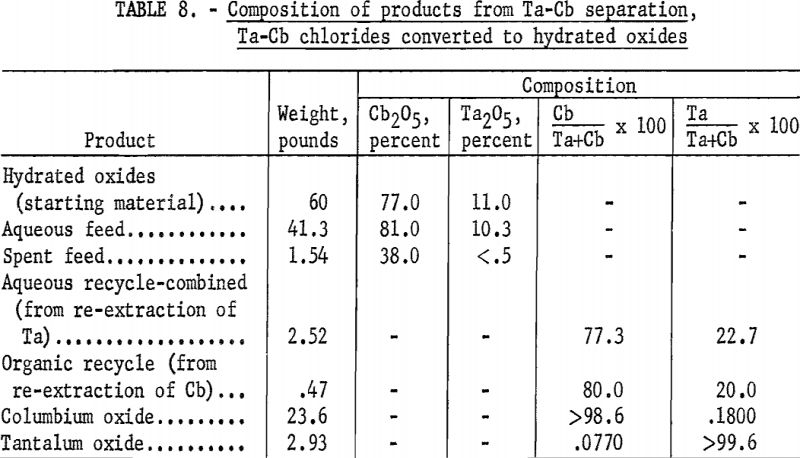
The apparatus and procedure used for this run were the same as those described for the preceding run. However, conditions of acid concentration and flow rates were changed to effect a higher recovery of the columbium product (table 7). To eliminate the stripping operation, the final tantalum product was precipitated directly from the organic solution, and collected on a filter. Results of this run are shown in tables 8 and 9.
Recoveries of 85.9 percent columbium and 80.4 percent tantalum were obtained. Again these figures were based upon the weight of tantalum or columbium oxides that passed through the system, excluding the amounts of oxides that remained in the mixer-settlers at completion of the run. If recycle solutions were returned to the separation system, as they would be in a long-term run, the estimated recoveries would be 98 percent columbium and 96 percent tantalum.
Purification of Rare-Earth Product
The third step in processing euxenite concentrate was separating and recovering the valuable metals contained in the chlorination residue. This product contained the chlorides of the rare-earth elements and of thorium, uranium, and iron, along with carbon and unreacted ore. The hydrometallurgical procedures used included separating carbon and unreacted ore from the soluble chlorides by filtration. Uranium, thorium, iron, and cerium were separated from the other elements by oxidation reactions and pH control. The resulting solution was treated with sodium sulfate to precipitate the light rare-earth elements. Finally the yttrium-rich, heavy-rare-earth fraction was precipitated as hydroxide.
Procedure and Results for Small-Scale Tests
Before the large-scale processing was undertaken, preliminary tests were made on a laboratory scale to establish the conditions to be used. A sample of the chlorination residue was heated to 300° C. in the presence of air to oxidize iron and uranium. Then it was dissolved in water, and the resulting solution was filtered to remove carbon and unreacted ore. The filtrate contained about 100 grams of oxides per liter. Ammonium hydroxide was added slowly to the filtrate, and iron, uranium, and thorium precipitated at a pH of approximately 3.5. The precipitate was filtered from the solution. Addition of dilute ammonium hydroxide was continued to the pH of incipient precipitation, approximately 4.8. Hydrogen peroxide was added to the solution to oxidize cerium (III) and precipitate it as ceric hydroxide. This precipitate was removed by filtration. Hydrolysis lowered the pH of the solution, which was returned to pH 4.8 by the addition of ammonium hydroxide; the solution was again treated with hydrogen peroxide. This procedure was repeated several times to insure complete removal of cerium.
For separating the rare-earth elements by double sulfate fractionation the concentration of the solution must be maintained at about 100 grams of rare-earth oxides per liter. The filtrate was evaporated until this concentration was obtained. A solution containing 100 grams per liter of sodium sulfate was added to the cold chloride solution. An insoluble double sulfate of sodium and the light rare-earth elements was obtained as fine crystals, which were separated from the soluble fraction by filtration. The precipitate was dissolved in acid, and the metals were reprecipitated as oxalates, which were ignited to oxides. The metals in the soluble rare-earth fraction were also precipitated as oxalates and treated in the same manner. Results of the fractionation showed that 14 percent of the rare-earth oxides were collected in the insoluble fraction, which contained 8.1 percent yttrium oxide, and 86 percent of the rare-earth oxides were collected in the soluble fraction, which contained 59 percent yttrium oxide.
Equipment
The equipment used for the large-scale work consisted of a 50-gallon, steam-jacketed reactor equipped with a polyethylene-coated agitator; a Shriver, 10-inch plate-and-frame filter press; and several 50-gallon, polyethylene-lined storage tanks.
Procedure and Results
One hundred and thirty pounds of chlorination residue was treated with 50 gallons of water to extract the soluble chlorides. Unreacted ore and carbon were separated from the solution by filtration. The pH of the filtrate was adjusted to 3.5 by adding ammonium hydroxide, and hydrogen peroxide was added to oxidize iron. The precipitate contained uranium, thorium, and iron hydroxides and was removed by filtration. The pH of the filtrate was raised to 4.8 by adding ammonium hydroxide. Hydrogen peroxide was used as an oxidizing agent, and the solution was heated to 70° C. The remaining uranium, thorium, iron, and some of the cerium were precipitated and collected by filtration. The filtrate from this step was treated with 38 pounds of sodium sulfate to convert the rare-earth chlorides to double sulfates. Lanthanum, cerium, praseodymium, neodymium, and samarium, the light rare-earth elements, which comprised the insoluble fraction, were filtered from the solution. The heavy rare-earth elements, gadolinium, dysprosium, and erbium, along with a major part of the yttrium, remained in solution as sulfates, and later were collected as hydroxides. The final products were dried at 65° C. Table 10 lists the quantities and composition of the four products collected.
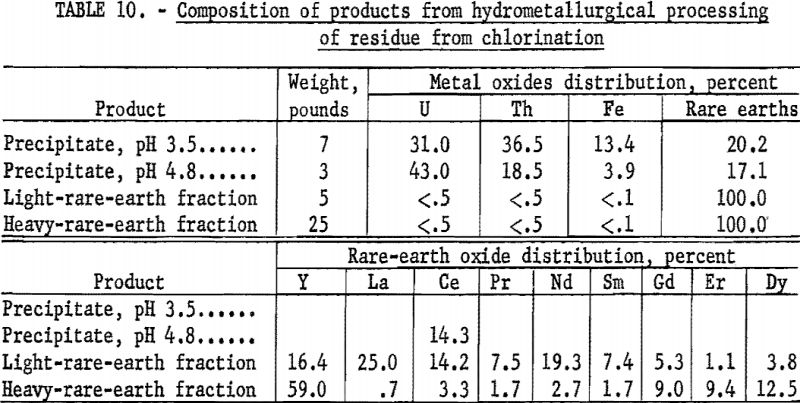
The yttrium oxide content of the heavy rare-earth-element fraction was 59 percent. Recovery of the yttrium collected in this fraction was calculated to be 102 percent of that present in the euxenite concentrate. Recovery of the total rare-earth elements combined in the light and heavy fractions was 101 percent of the quantity of these elements in the starting material.
Purification of Uranium Product
Separation of the metals contained in the eutectic product from the chlorinating run was the final step in processing euxenite concentrate. This eutectic product weighed 44 pounds, was equivalent to 20.7 pounds of oxides, and contained 64.4 percent uranium, 32.6 percent iron, and 1.4 percent combined tantalum and columbium oxides. Before the large-scale processing of the eutectic, tests in the laboratory determined suitable operating conditions. A sample of eutectic was dissolved in water and treated with sodium carbonate, producing a soluble uranium complex and insoluble ferric hydroxide. The carbonate complex was then destroyed, and the uranium was precipitated as a hydroxide.
Equipment
Equipment for large-scale processing included a muffle furnace in addition to the apparatus described for the previous hydrometallurgical processing.
Procedure and Results
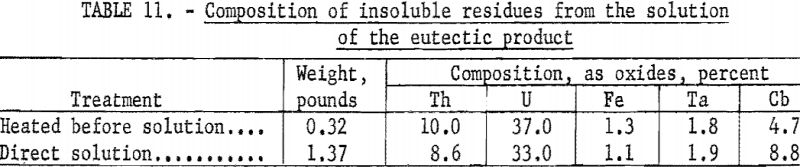
Two procedures were used to dissolve the 44 pounds of eutectic product. In one, 21 pounds of the material, was heated in a muffle furnace at 300° C. for 2 hours, and then dissolved in 6 gallons of water by mixing with a power stirrer for 48 hours. The insoluble residue was filtered from the solution. In the alternate procedure, the remaining 23 pounds was treated directly with water, which dissolved most of the material readily, although a colloidal suspension was noted. The insoluble residue was collected. Filtrates from the two dissolution procedures were combined before further processing. The quantities and compositions of the insoluble residues from the two procedures are given in table 11.
To separate iron and uranium contained in these combined filtrates, sufficient sodium carbonate was added to the solution to make it basic. Uranium was converted to a soluble complex, and iron precipitated as ferric hydroxide, which was removed by filtration. The filtrate then was heated to 70° C. for 8 hours in the presence of hydrochloric acid to destroy the carbonate complex. Ammonium hydroxide was added, and the uranium precipitate was collected by filtration.
The quantities and composition of the resulting products are given in table 12. The uranium product contained 33.5 percent of the uranium present in the eutectic, which is equivalent to 22.2 percent of the uranium in the euxenite concentrate.