Table of Contents
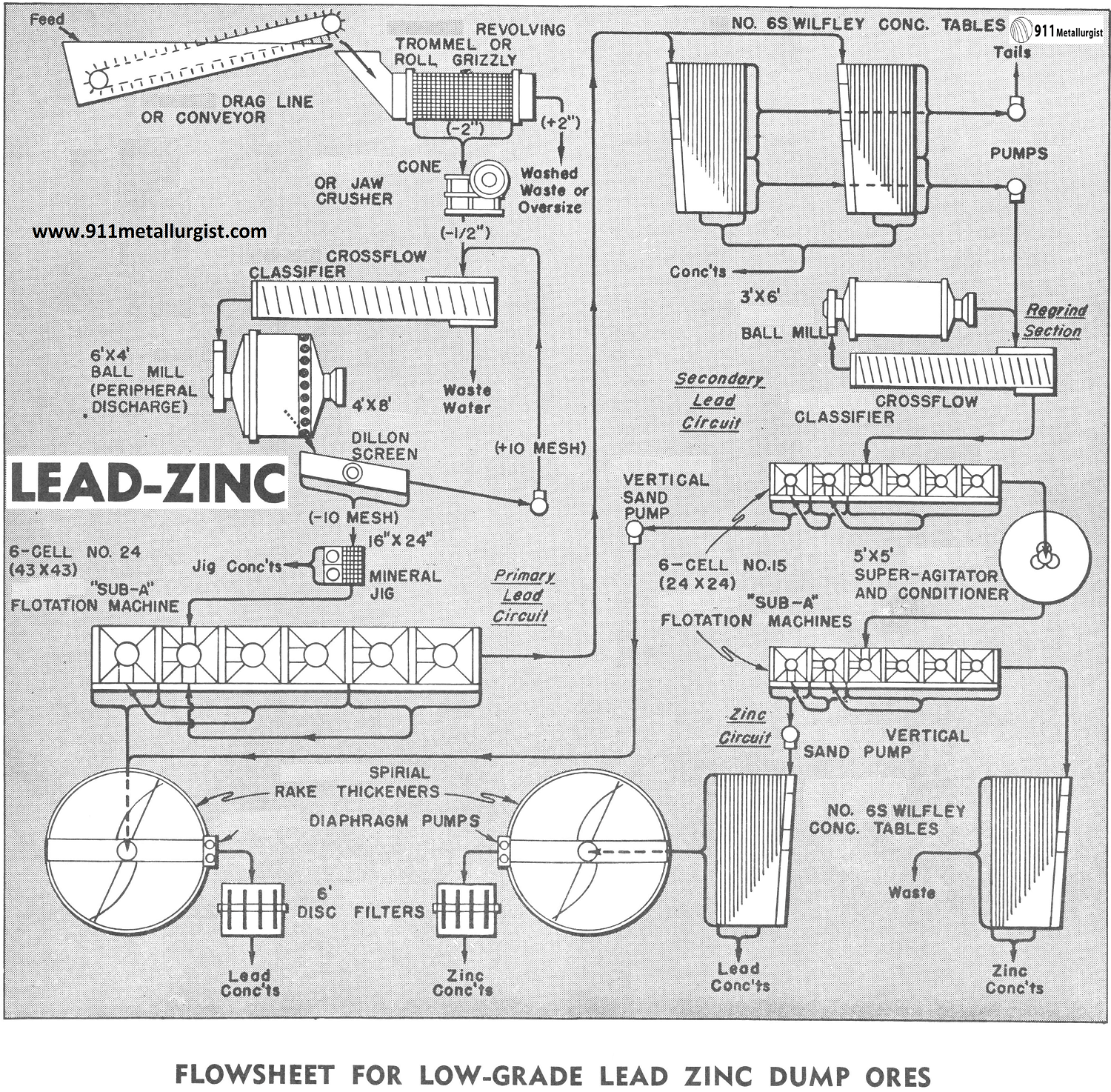
The flowsheet above was designed to treat economically approximately 200 tons in 24 hours of a low-grade dump ore containing lead and zinc values as well as some silver in both sulphide and oxide form. Due to varying specific gravities of the values, gravity concentration in addition to flotation was indicated. As is common in many such cases, the dump contained material over 2″ in size as well as some debris. This necessitated an arrangement for scrubbing and separating this coarse material at the head of the circuit.
Lead and Zinc Pb-Zn Separation Process Circuit
Primary Washing and Crushing
A Drag or Conveyor is used to bring the dump material to a Revolving Trommel Screen which discards all plus 2″ material and debris. A thorough washing and some disintegration is given the ore by the trommel screen. The minus 2″ material passes to a cone or Forced Feed Jaw Crusher and is reduced to approximately minus ½”. A Cross-Flow Classifier is used to dewater this minus ½”ore and also removes fine colloids. Classifier overflow is discarded.
Lead Ore Grinding Circuit
The dewatered minus ½’ ore from the classifier feeds to a 6’x4′ Peripheral Discharge Ball Mill. This type of mill was selected to produce a minus 10 mesh product for jigging with a minimum of overgrinding. A 4’x8′ Vibrating Screen separates the ball mill discharge at 10 mesh, the oversize returning to the classifier for additional grinding. The minus 10 mesh screen product passes over a 16″x 24″ Duplex Mineral Jig. This unit recovers not only a lead-silver concentrate in sulphide form but also recovers a high percentage of the lead carbonates and lead oxides.
Lead Flotation and Tabling
The jig discharges into a 6 cell No. 24 “Sub-A” Flotation Machine which produces a high-grade lead concentrate which is dewatered in a Thickener. A Adjustable Stroke Diaphragm Pump discharges the thickener underflow into a 6′ Disc Filter for further dewatering of the final lead concentrate. Flotation tails are fed to two Wilfley Concentrating Tables for additional recovery of the oxide values. These tables produce a final tailing (which is pumped to waste by a SRL Sand Pump), a lead concentrate and also a middling product containing both lead and zinc.
Regrind Circuit
The table middlings are pumped by a SRL Sand Pump to a regrind circuit consisting of a 3’x6′ Ball Mill, a 24″ CrossFlow Classifier and a 6 cell No. 15 “Sub-A” Flotation Machine. The classifier overflows a product at approximately 80 mesh directly to the flotation machine where a cleaned and recleaned lead concentrate is combined with the concentrate of the 4 cell lead flotation machine.
Zinc Flotation
Tailings from the 6 cell regrind flotation machine contain the zinc values which are conditioned in a 5’x5′ Super-Agitator and Conditioner. The zinc is cleaned and recleaned in a 6 cell No. 15 “Sub-A” Flotation Machine. The zinc tailings pass over a Wilfley Table which gives the operator a constant, visual picture of the efficiency of the zinc flotation machine, and also recovers additional zinc values.
Zinc concentrates are fed to a Wilfley Table to remove any lead oxide values which may have floated with the zinc, and then are thickened in a Thickener. A Adjustable Stroke Diaphragm Pump takes the thickener underflow to a 6′ Disc Filter.
SUMMARY
This flowsheet takes advantage of recovering lead minerals at a coarse size not only by means of a Mineral Jig but also by utilizing the ability of the “Sub-A” to handle a coarse feed. The two pilot tables, taking the coarse flotation tailings, cut down the tonnage fed to the regrind circuit.
One of the greatest difficulties in any flowsheet is the treatment of the middling product. Early removal of the slimes and the use of a regrind circuit greatly simplifies the problem of middlings in this mill.
Flotation of Lead Ore
By far the most important lead mineral is the sulphide, galena. In many of the ores in which it occurs it is associated with sphalerite or marmatite, and two-stage selective flotation is necessary to separate the two classes of minerals. When, however, the lead mineral is the only one of importance—and such ores are not uncommon—it is usually extracted by gravity concentration in jigs and on tables, followed, after thickening and grinding if necessary, by the flotation of any middlings and tailings that are too high in lead values to be sent to waste; direct flotation is comparatively rare except for the treatment of tailing dumps.
The flotation of galena Pb is comparatively simple. It is usually carried out in subaeration machines, presumably because the cells provide a ready means of high-speed conditioning ; the proper pre-treatment with the necessary reagents of the various products as they come from the different parts of the gravity concentration plant might otherwise be none too easy. Single or double cleaning of the rougher concentrate of the flotation section is generally necessary, this operation often being done in pneumatic cells. Because of the variations in the flow sheets of gravity concentration plants, it is impossible to indicate the points at which the different reagents should enter the circuit, but their normal consumption is usually within the following approximate limits:

Pine oil is not often employed as it tends to produce too stiff a froth ; cresylic acid is preferred, reinforced, should the froth be too delicate, with a coal-tar creosote, or, more rarely, with pine-tar oil. The usual promoters are ethyl and amyl xanthates, either alone or in combination, or else aerofloat, either alone or with a small quantity of one of the dithiophosphates. Soda ash is commonly employed for maintaining the alkalinity of the circuit, but lime is replacing it to some extent, especially in those plants where amyl xanthate or aerofloat reagents are used. Sodium silicate is sometimes added as a deflocculator when the gangue has too much tendency to enter the concentrate. Should the ore contain an appreciable quantity of sphalerite, though not enough to warrant the production of a separate zinc concentrate, it can usually be depressed by the addition of sodium cyanide with or without zinc sulphate either to the grinding circuit or to a conditioning tank. It is not often that more than 0.1 lb. per ton of either reagent is required for this purpose.
The oxidized lead minerals, of which cerussite and anglesite are the most important, can generally be concentrated by flotation after their surfaces have been coated with a film of lead sulphide by a preliminary sulphidizing treatment. Since the consumption of the sulphidizing reagent is proportional to the surface area to be attacked, it is usual, particularly with a rich ore, to table out as much coarse mineral as possible before flotation. The table tailings, reground if required to a suitable size, are treated with soda ash to precipitate any dissolved salts which might react with the sulphidizing reagent, and with sodium silicate in addition, should it be necessary to deflocculate the gangue. The pulp is then conditioned with sodium sulphide, or, more rarely, with one of the other alkaline sulphides, the normal range of consumption being 2 to 6 lb. per ton of ore. The sulphidizing contact period may be as long as 20 minutes, but in many cases it has been found possible to reduce the time to a few minutes, especially when flotation is carried out in subaeration machines, the cells of which have the advantage that additional reagent can be introduced at any point if extra sulphidizing is needed. The minerals are floated with frothing and promoting reagents similar to those required for galena, amyl xanthate being very effective with this class of mineral.
Source: This article is a reproduction of an excerpt of “In the Public Domain” documents held in 911Metallurgy Corp’s private library.
