Table of Contents
In separating and purifying rare-earth compounds, ion-exchange and liquid-liquid extraction techniques have exhibited the greatest potential. Of the two, ion exchange has received major attention and has been exploited into commercial operation. Notable research has been reported on solvent extraction techniques, especially in extraction of tetravalent cerium with phosphates and phosphonates. Work along this line usually was done with pure solutions or, in a few instances, with artificial mixtures of pure rare-earth compounds. A more pertinent problem is extraction of tetravalent cerium from a mixed solution prepared from an ore or concentrate.
For the present study, a sulfate solution of mixed rare-earth elements prepared from a bastnasite concentrate was chosen. The sulfate ion is known to inhibit tetravalent cerium extraction, and it was an aim of the project to see whether its effect could be overcome. This goal was achieved on a separatory funnel scale.
Having extracted the tetravalent cerium into the organic phase, in this case into TBP diluted with benzene, the next step is to back extract into another aqueous phase. This generally is done by first reducing the cerium with hydrogen peroxide and then stripping with water. This method has two disadvantages: (1) In any continuous system the hydrogen peroxide would tend to be carried via the organic phase to the feed solution and cause undesirable reduction; and (2) in a pressurized system decomposition of the hydrogen peroxide causes pressure disruptions. This latter effect interfered with the flow of the organic phase. A search for a nonreducing stripping agent resulted in use of a dilute sulfuric acid solution.
Having the cerium in the sulfate strip solution, several techniques other than the conventional and costly oxalic acid precipitation were tried for removing cerium from the strip solution. Three precipitants, sodium hydroxide, sodium sulfate, and ethanol, gave complete precipitation.
Preliminary work was done on a separatory funnel scale, but a considerable amount was also done using the pressure-bubbler equipment. This equipment, basically a series of spray towers, performed well, except that the relatively long contact time of the TBP and highly acid feed solution resulted in reduction of tetravalent cerium in the feed; however, the pressure-bubbler showed promise for application to this type of extraction.
The efficiency of amines for separating rare-earth elements and fractions from acidic sulfate solutions also was investigated.
Amines have received considerable attention as extractants for uranium. Primary and tertiary amines have also been used for separating rare-earth elements from sulfate and nitrate solutions. From these reports and the present study, amines have definite possibilities as trivalent rare-earth extractants.
Experimental Procedures
Tetravalent Cerium
The rare-earth sulfate solution was prepared by reacting the bastnasite concentrate with sulfuric acid, roasting at 1,200° F, to expel the excess acid and render the gangue materials insoluble, and finally water leaching.
Nitric acid was added to the sulfate solution to form the complex (Ce(NO3)4=, plus some excess to prevent hydrolysis and hence precipitation.
The cerium solution subsequently was oxidized to the tetravalent state electrolytically, using platinum electrodes with a large anode-to-cathode surface area (fig. 1). The oxidation usually was carried out simultaneously in several such cells connected in series. Generally, it was not feasible to oxidize more than 96 percent of the cerium, because of the large drop in current efficiency.
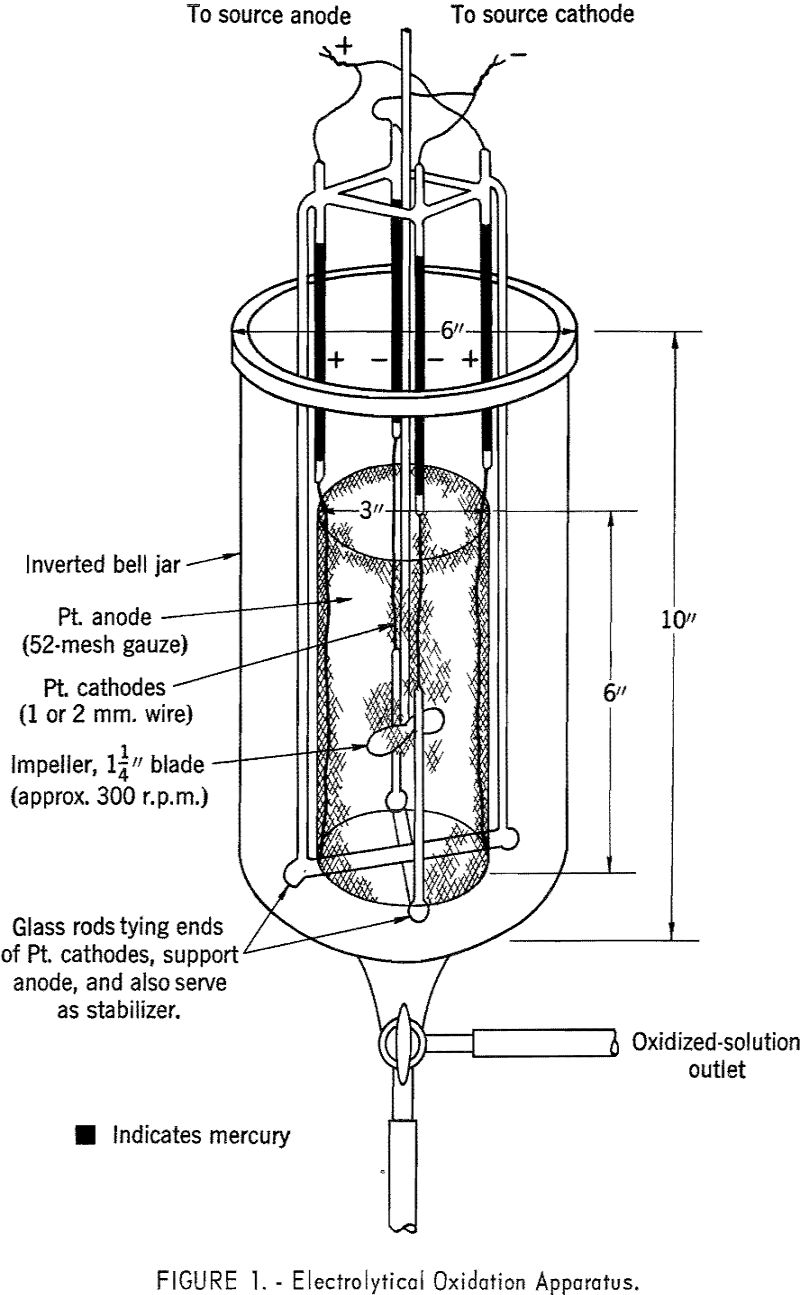
Operating at room temperature with 30-second shaking time and separating the phases immediately after disengagement produced optimum results in separatory funnel experiments.
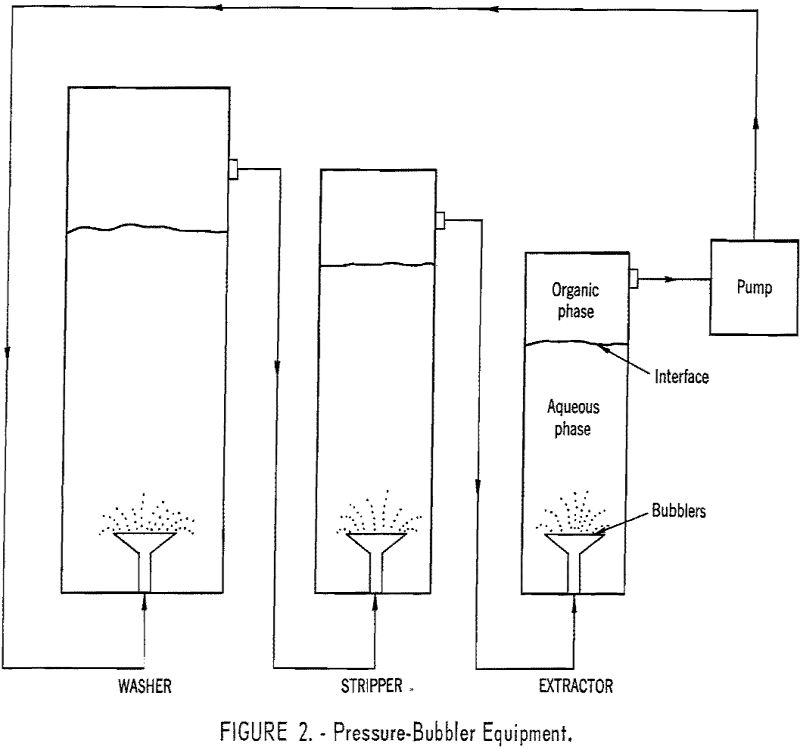
The pressure-bubbler equipment (fig, 2) consists of three spray towers: (1) Extractor; (2) washer; and (3) stripper. The lower layers in each vessel are the continuous, aqueous phase. The upper layers are the organic phase, TBP in benzene. In operation the organic phase bubbles through the three vessels in closed circuit as follows:
- Through the extractor, picking up tetravalent cerium, nitric acid, and some trivalent rare-earth elements.
- Through the washer, where most of the trivalent rare-earth elements and nitric acid are dropped.
- Through the stripper where the tetravalent cerium is removed.
Trivalent Rare-Earth Elements
The equipment and procedures in the amine experiments were generally standard funnel techniques, except for use of a modified small continuous
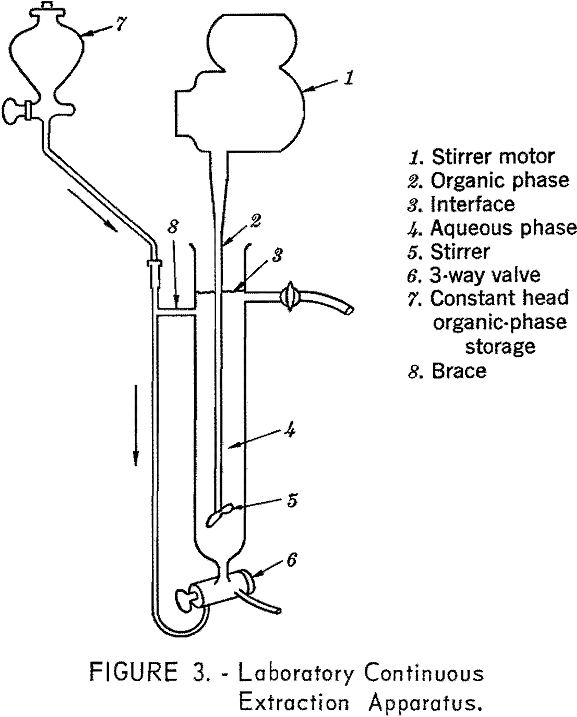
extractor originally designed by Bewick, Currah, and Beamish (fig. 3). In this apparatus the aqueous phase is mechanically stirred while the organic phase passes through it and is taken off at the top. The withdrawn organic phase can be separated into discrete fractions and analyzed separately.
Analyses
Total cerium and oxidized cerium and acid concentration were determined volumetrically. Other earth oxides were determined by X-ray fluorescence on samples precipitated as the oxalate and roasted to the oxide.
Experimental Results
Tetravalent Cerium
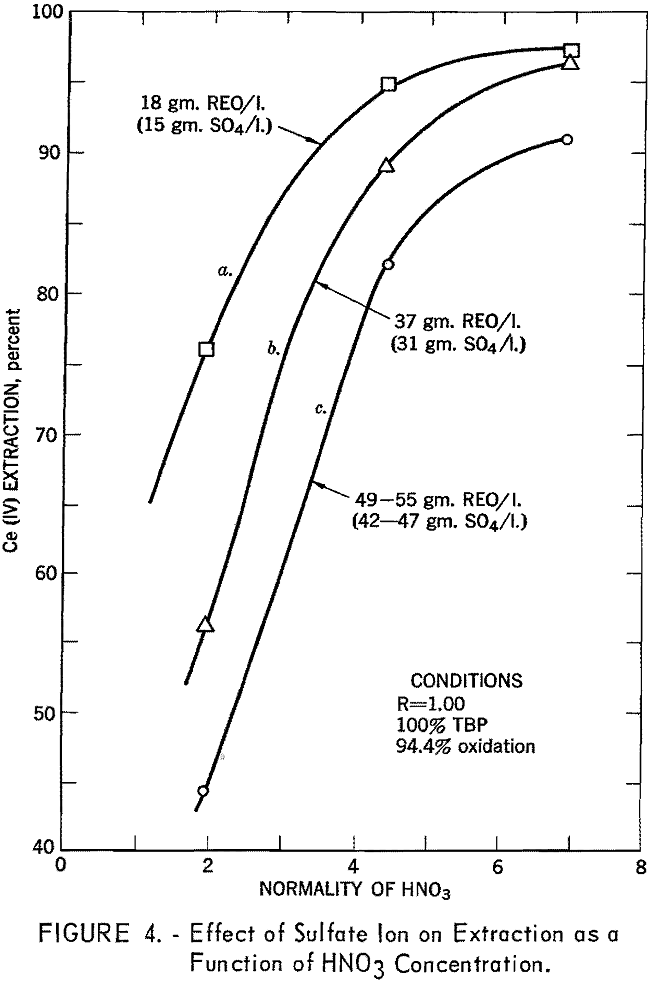
Although the extraction of tetravalent cerium from pure solution is independent of acid concentration, such is not the case when the sulfate ion is present. In the solutions prepared from the bastnasite concentrate, it was necessary to have at least 6 N nitric acid to achieve satisfactory extraction (fig. 4). In subsequent work the feed was adjusted to 7.5 N nitric acid, which allowed substantially complete extraction.
In the separatory funnel work 100-percent extraction of the tetravalent cerium was achieved using equal volumes of the aqueous phase (7.5 N nitric acid, 25 g. CeO2/1.) and organic phase (50 percent TBP in benzene).
Three solutions were found to give 100-percent back extraction of the tetravalent cerium from the organic phase. These were potassium carbonate, sodium sulfate, and sulfuric acid solution. The first two usually resulted in precipitation during the back extraction. The dilute sulfuric acid did not cause precipitation and is the more desirable as a nonreducing stripping agent.
Other than oxalic acid, three materials were found to precipitate the cerium efficiently from the sulfuric acid strip solution. These were sodium hydroxide, sodium sulfate, and ethanol. The latter two required adjustment of the solution to a pH of about 3 before precipitation. The sodium sulfate reaction also required heating to insure complete precipitation. After establishment of the basic variables on a separatory funnel scale, the extraction technique was tried, using pressure bubbler equipment. It was found that there was no exact correlation between the two systems.
The use of either potassium carbonate or sodium sulfate solution for stripping proved impossible, because the precipitates formed entrained in the organic phase and clogged the transfer lines and valves. A series of tests were then made, using sulfuric acid at different concentrations for stripping. A 10-percent solution was found best.
There still existed a problem of entrainment and interface precipitation in the wash vessel. A series of tests were made, using different nitric acid concentrations in the washer in place of the water previously used. A 5-percent nitric acid solution eliminated the problem.
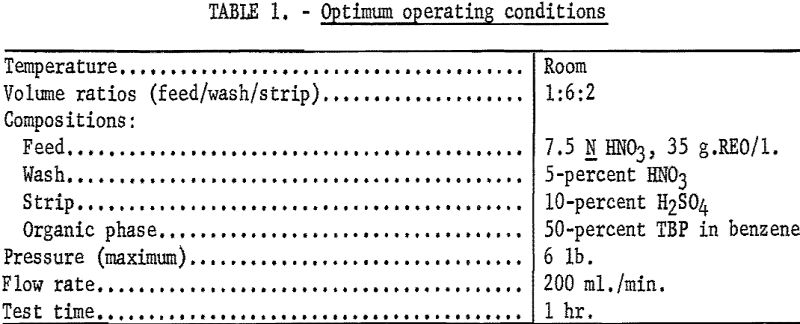
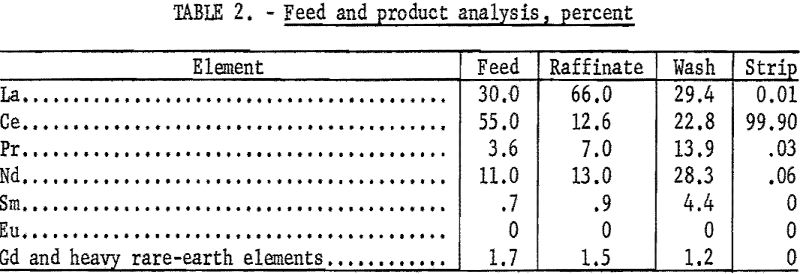
In the pressure bubbler, a maximum of 86 percent recovery of 99.7-percent cerium in the sulfate strip solution was achieved. The best operating conditions are summarized in table 1, and the feed and product analysis of the various solutions are given in table 2.
Trivalent Rare-Earth Elements
Preliminary separatory funnel tests using 0.2 M amine in an immiscible organic liquid and an equal volume of 0.2 M rare-earth sulfate solution (pH 1) showed that the primary amines gave the highest extraction of rare-earth elements. A study of separation factors gave results that varied from 1.2 for Nd-Pr pairs to 12 for Ce(III)-Yb(III) pairs.
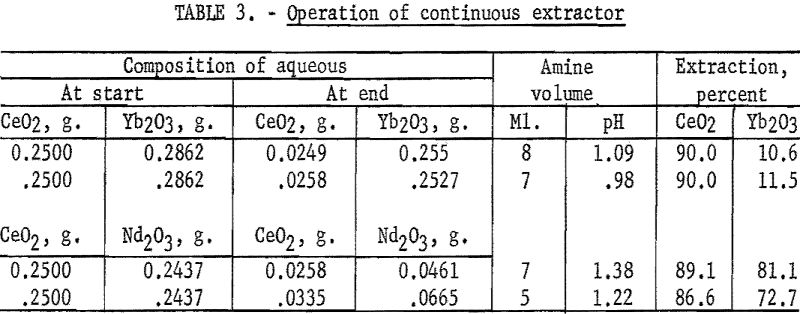
The study was extended in the continuous reactor previously described. In this series of tests Primene 81R was dissolved in isopropyl ether and passed through sulfate solutions of the pairs Ce(III)-Yb(III) and Ce(III)-Nd (table 3). These pairs were chosen because they gave widely differing separation factors. By extending the number of extractions of the Ce(III)-Yb(III) pair, a 98+ percent ytterbium fraction was produced.
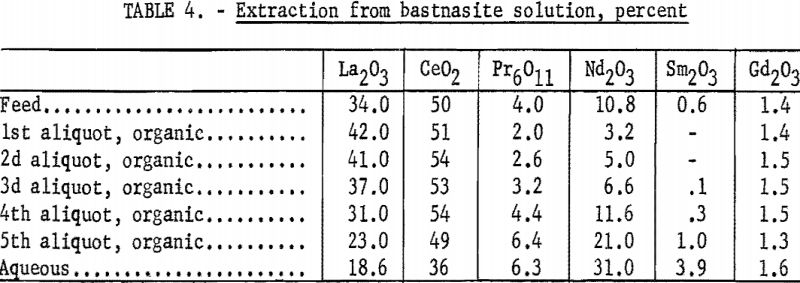
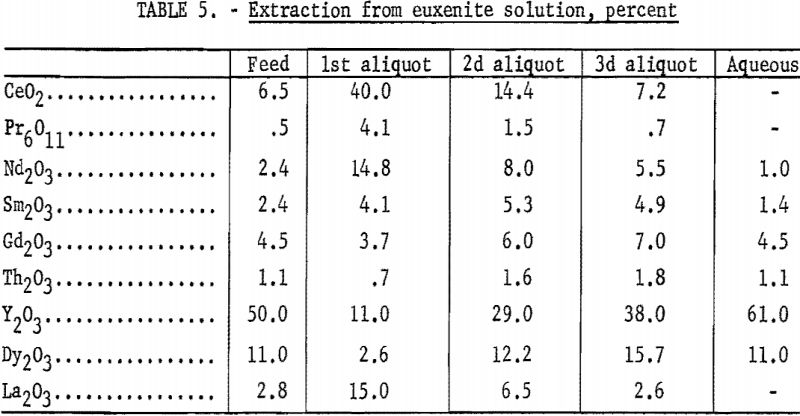
The continuous extractor was applied to sulfate solutions prepared from a bastnasite concentrate (table 4) and from a euxenite carbonate residue (table 5). The cerium-group elements extracted more readily than did the yttrium group. The greater separating efficiency obtained from the euxenite carbonate residue was due to its smaller percentage of cerium-group rare-earth elements and its larger spread in atomic weights. The amount of cerium and lanthanum present affects the direction of change of separation factors of heavier rare-earth elements.
Discussion and Conclusions
On a separatory funnel scale, TBP efficiently extracts tetravalent cerium from solutions containing the sulfate ion, providing the nitric acid concentration is 6-8 N. The high nitric acid concentration, dictated by the sulfate ion, increases processing costs and intensifies an already serious corrosion problem.
Formation of precipitate, although undesirable in any continuous operation, might be tolerated in a batch operation, since it is the usual case to produce a solid product. In such instances, potassium carbonate or sodium sulfate strip solutions might be useful. The sulfuric acid strip solution, however, is more convenient to handle and will back extract 100 percent of the cerium from the organic phase.
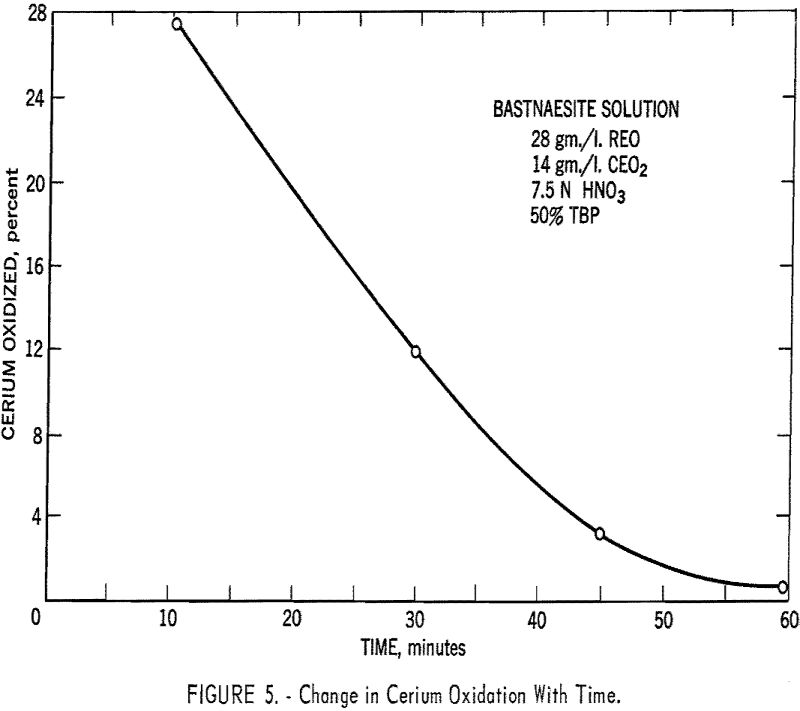
The technique is not as effective using the pressure bubbler, due to reduction of cerium in the feed (fig. 5) by the decomposition products of the TBP, which, in turn, result from the action of the strong nitric acid on the TBP. This difficulty did not arise during the separatory funnel work because of the much shorter contact time. It is necessary, therefore, to do away with the high nitric acid concentration in the pressure-bubbler equipment. Either the sulfate ion will have to be removed beforehand, or its deleterious effect eliminated by some means, such as nonextractable complex formation.
The pressure-bubbler equipment works quite well. This type of equipment has not found any widespread use, mainly because of its low volumetric efficiency. It does, however, have its own advantages as follows;
- Simplicity, referring to an internal structure having no packing, plates, stirrers, or baffles.
- Low organic phase loss, because of nonviolent mixing action.
- Versatility and ease of control are inherent in the simplicity of the equipment.
- The equipment has possibilities for continuous as well as batch use.
The recovery of the cerium from the sulfuric acid strip solution presents no problem. If the use of oxalic acid is undesirable, then any of the other three precipitants should be satisfactory, with ethanol showing the greater promise.
Although much work remains to be done, primary amines show promise for separating rare-earth mixtures in sulfate solutions. This system appears to have some advantage over the TBP-nitric acid system, both cost wise and in regard to corrosion problems, because of its lack of a high HNO3 concentration and of the corrosion-inhibiting effects of amines.
