The growth in demand for certain lanthanides has resulted in the development of industrial processes for their separation and purification from source materials. These operations have resulted in the availability of substantial quantities of a byproduct mixture of rare-earth elements that contain mostly samarium and neodymium, and lesser amounts of praseodymium, cerium, and lanthanum. Potential uses for samarium-cobalt alloys have generated an interest in developing methods for recovering samarium from these source materials.
Continuous countercurrent solvent extraction systems have received attention as the most economical and efficient methods for producing pure lanthanides in quantity. The success of these systems depends mainly on the separation factors between the desired lanthanide and adjacent lanthanides being large enough to permit purification in a reasonable number of stages. The absence of europium and promethium (atomic numbers 63 and 61, respectively) , from the byproduct rare-earth mixture containing samarium (atomic number 62) renders it an excellent feed stock for samarium separation by solvent extraction.
Research conducted using di(2-ethylhexyl) phosphoric acid (EHPA) has demonstrated the feasibility of separating meodymium from samarium using this organic extractant. Separation factors in the range of 7 to 10 for samarium/neodymium (Sm/Nd) were obtained using aqueous feed concentrations of <0.5 molar samarium and neodymium. These separation factors are sufficiently large to suggest the possibility of separating this pair of lanthanides in a reasonable number of stages in a counter current system. The commercial availability of EHPA also favors adoption of this system.
The mechanism of extraction by EHPA has been studied in detail by other investigators. The extracting species is a mono-ionized dimer of EHPA, and three of these anions extract one trivalent metal cation. Designating the EHPA anion as G-, the mechanism of extraction may be written as

This mechanism prevails up to the ratio of 1 mole of metal ions to 3 moles of EHPA dimers (6 moles of EHPA). When this ratio is exceeded by adding more metal ions, the second acidic hydrogen in some of the dimers becomes displaced, allowing cross-linking of the dimers. This yields a gelatinous polymeric species that has limited solubility in the organic diluent.
This study focused on developing a system to obtain high recovery of >99-pct-pure Sm2O3 without gel formation in the organic phase. The possibility of overloading the organic phase was avoided by initially extracting all of the rare-earth elements into the organic phase under conditions that insured a low ratio of rare-earth elements to EHPA. Separation was accomplished by selectively stripping rare-earth elements from this loaded organic phase. Both single- and multiple-stage systems were investigated.
Nomenclature
Distribution coefficient.-Ratio of the concentration of a lanthanide in the organic phase to its concentration in the aqueous phase.
Separation factor.—Ratio of distribution coefficients of the lanthanides.
Mass transfer.-Ratio of the mass of the lanthanides in the aqueous acid strip solution to the total mass of the lanthanides in the system.
Percent extraction.-Ratio of the mass of the lanthanides in the organic phase to the total mass of the lanthanides in both phases multiplied by 100.
Percent recovery.—Ratio of the mass of a particular lanthanide in the aqueous acid strip solution to the total mass of that lanthanide in the system multiplied by 100.
Phase ratio.—Ratio of the volume of the organic phase to the volume of the aqueous phase.
Flow ratio.—Ratio of the flow rate of the organic phase to the flow rate of the aqueous phase.
Materials and Procedure
The rare-earth mixture used in this study was a byproduct of bastnasite processing; the europium, lanthanum, and cerium had been substantially removed. The composition of the mixture is shown in table 1.
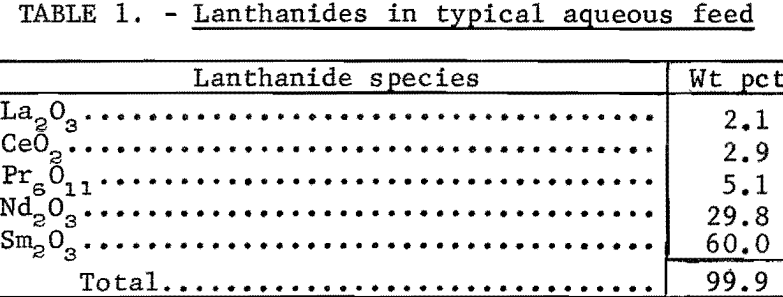
The initial aqueous feed was prepared by dissolving the rare-earth mixture in diluted nitric acid and adjusting the solution to the desired pH by adding ammonium hydroxide. All lanthanide concentrations are reported as grams of rare-earth oxide (RE2O3) per liter of solution.
The organic phase was prepared by dissolving the desired vol pct of extractant in Socal 355L, a commercially available petroleum fraction containing 55 pct aromatics, 18 pct paraffins, and 27 pct naphthenes. Other diluents were also investigated briefly.
For single-stage studies, the organic phase was contacted with the aqueous feed in a separatory funnel at a phase ratio and pH such that the aqueous phase was depleted of substantially all of its rare-earth constituents. The standard aqueous feed was 280 to 373 g RE2O3/l, and the standard organic phase was 50 pct EHPA in Socal 355L. After separating the two phases, the loaded organic phase was contacted with the stripping solution, and the pH of the equilibrated aqueous stripping phase was adjusted to the desired value by adding acid or base. The lanthanides were precipitated from the separated aqueous phase at pH 3 using oxalic acid. Lanthanides remaining in the organic phase were stripped with 6 N HCl and precipitated at pH 3 using oxalic acid. The oxalates were ignited to produce oxides at 1,000° C, weighed, and analyzed by X-ray fluorescence.
Multistage experiments were conducted in a 20-stage mixer-settler, in which each mixing chamber had a net volume of 10 ml, and each settling chamber, a net volume of 50 ml. Flow rates ranged from 8 to 25 ml/min, and organic-to- aqueous- flow ratios ranged from 2:1 to 1:1. Loaded EHPA for the mixer-settler experiments was prepared by contacting the organic phase with the aqueous feed in a large cylindrical vessel using mechanical stirring. The pH was raised by adding a mixture of ammonia gas and air through a gas dispersion tube immersed in the organic phase. After running this organic phase through the selective stripping mixer-settler unit, the lanthanides (mainly samarium) remaining in the organic phase were removed by contacting the EHPA with 6 N HCl in a second mixer-settler unit. The barren organic phase was returned to the cylindrical contact vessel for recycling. No deterioration of the EHPA was detected even after 10 cycles.
Samples were obtained during mixer-settler runs by collecting all of the organic and aqueous effluents over a specified time interval. These samples were processed by the same techniques used in the single-stage studies.
Results and Discussion
Evaluation of Strip Solutions
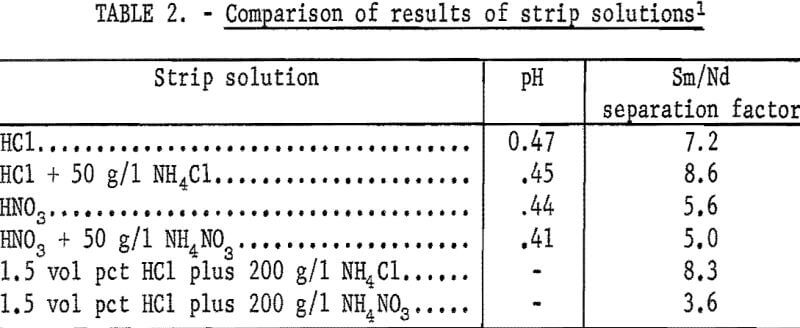
Hydrochloric acid and nitric acid were investigated as possible stripping agents for the loaded EHPA. The initial aqueous strip solution would be barren of metal salts and would, therefore, have a relatively low density, almost as low as the density of the organic phase. This lack of difference in densities of the two phases would cause slow phase disengagement. To avoid this, amnionium salts were added to the strip solution to increase its density. The effect of adding salts to the stripping solution was investigated, with particular attention paid to the separation factor for Sm/Nd.
As shown in table 2, the use of a hydrochloric acid stripping solution results in the highest separation factors. The data indicate that adding ammonium chloride to the stripping agent enhances separation factors obtained using the chloride stripping system. Separation factors obtained using the nitrate system remained essentially constant when ammonium salt was added.
Sulfuric acid was not investigated as a stripping agent because lanthanide sulfates have limited aqueous solubility that decreases as the temperature increases. This characteristic makes sulfate systems difficult to control.
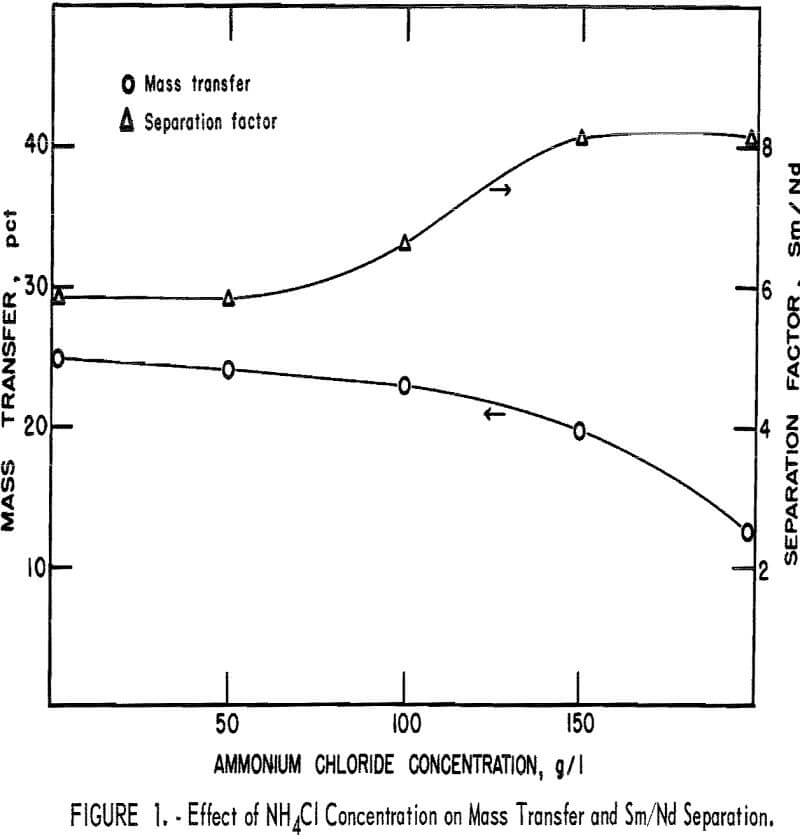
Effect of NH4Cl Concentration on Mass Transfer and Separation
The concentration of ammonium chloride in the HCl strip solution was investigated to determine its effect upon mass transfer and the Sm/Nd separation factor (fig. 1). Experiments were conducted under constant conditions using an organic phase consisting of 50 pct EHPA in Socal 355L and containing 29 g RE2O3/l. The phase ratio was 1.5, and the equilibrium pH was 0.6. Mass transfer decreased from 24.5 pct with no ammonium chloride present, to 13.5 pct with 200 g/l NH4Cl. The Sm/Nd separation factors increased to a maximum at 150 g/l NH4Cl, and declined slightly on further addition of NH4Cl. Separation factor data was shown to be reproducible in a series of experiments. The decline in separation factors between 150 g/l and 200 g/l NH4Cl was negligible; however, the higher density of a strip solution containing 200 g/l NH4Cl was desirable for improving phase disengagement in the settlers of a counter-current apparatus. The strip solution selected for further study was 1.5 vol pct hydrochloric acid solution containing 200 g/l NH4Cl.
Effect of Organic Diluent on Mass Transfer and Separation
A major factor influencing the rate of phase disengagement in a solvent extraction system is the nature of the organic diluent. Various organic diluents were investigated, and their effects on mass transfer and Sm/Nd separation factor were determined. The results of these experiments are summarized in table 3. Experimental conditions consisted of 50 pct EHPA in diluent, 29 g RE2O3/l in organic phase, 200 g NH4Cl/l of strip solution at pH 1.0, and a phase ratio of 1.
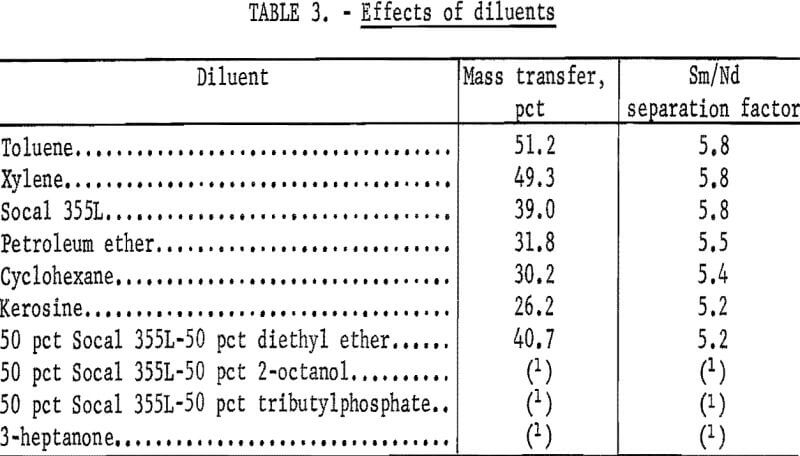
Use of toluene and xylene as diluents resulted in greater mass transfer than did Socal 355L, but the Sm/Nd separation factors were essentially iden- tical for all three diluents. Cost considerations eliminated toluene and xylene; therefore, Socal 355L was used in subsequent experiments.
Effect of pH on Mass Transfer and Separation
The effect of pH on Sm/Nd separation was investigated for selective stripping systems containing 50 pct EHPA in Socal 355L, 29 g RE2O3/l of the organic phase, and an aqueous HCl strip solution containing 200 g NH4Cl/l (fig. 2). The separation factor was found to decrease approximately from 9.5 to 8.0 as the pH was increased from 0.4 to 0.7. The use of a lower pH value further reduces the possibility of polymerization or gel formation and consequent flow interruption in a countercurrent operation. The data indicate that the pH in an operating system would have to be coordinated with other major parameters to insure optimum mass transfer, but, based on the curve presented in figure 2, effective separation of samarium from neodymium is possible over the entire pH range investigated.
Temperature Effect on Sm/Nd Separation
The mechanism of extraction of metal ions by EHPA suggests the presence of a chelated metal-organic species in the organic phase. Temperature may play an important role in the selectivity of a chelating agent due to strain in the metal-organic ring structure. The effect of temperature on the Sm/Nd separation factor for selectively stripping rare-earth elements from EHPA by HCI-NH4Cl was investigated; it was found that the separation factor decreased from 9.7 at 0° C to 7.8 at 60° € (fig. 3). This decrease appeared to be very close to a linear relationship over the temperature range studied. At ambient temperature, the Sm/Nd separation factor was ≈8.6 . The difference between this value and the maximum separation factor obtained was insufficient to

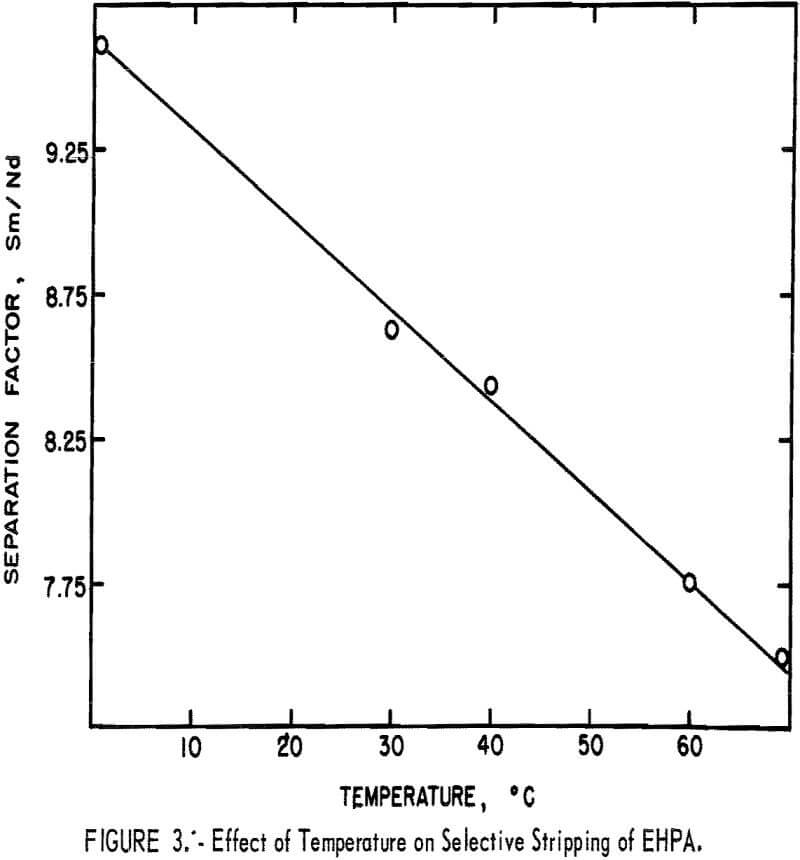
justify the difficulties and expense associated with the conduct of solvent extraction operations at temperatures other than ambient.
Countercurrent Mixer-Settler Studies
A number of countercurrent selective stripping experiments were conducted using the 20-stage mixer-settler under selected conditions of pH and flow rate. The organic phase contained 50 pct EHPA in Socal 355L and 29 g RE2O3/l, and the aqueous strip contained 200 g NH4Cl/l. Analysis of the aqueous and organic effluents at intervals ranging from 15 min to 4 hr indicated a direct correlation between percent mass transfer of the lanthanides and purity of the Sm2O3 remaining in the organic phase. Table 4 shows the results of these experiments. The experiment in which 76 pct of the Sm2O3 was recovered as 99-pct-pure Sm2O3 was conducted using effluent samples collected continuously over a 4-hr period after the system had reached equilibrium. All other samples were collected during an interval of 15 to 30 min after equilibrium had been obtained. Gadolinium was the major contaminant in the product of both runs that resulted in production of 99-pct-pure Sm2O3. The amount of Gd2O3 in the original feed was too small to detect, but it was upgraded along with the Sm2O3 to almost 1 pct in the product.

The operation of the mixer-settler unit under selective stripping conditions proceeded without the formation of any polymeric emulsion. Steady flow of both phases was maintained in the unit; minimal attention was required to prevent flooding and to maintain constant effluent concentrations and compositions.
Conclusions
The use of selective stripping techniques for rare-earth separation using EHPA solvent was shown to eliminate problems encountered in forward extraction systems. The addition of ammonium chloride to the hydrochloric acid stripping agent improved the physical properties of the system and also increased the Sm/Nd separation factor. The feasibility of using a continuous, countercurrent sequence to obtain the favorable recovery of >99-pct-pure Sm2O3 was demonstrated.
