Table of Contents
For the past two decades, about one-third of the copper consumed in the United States has been imported. In order to meet the Nation’s demand, new and improved methods of copper extraction must be found. Ferrothermic extraction is one possibility; the Bureau of Mines undertook this investigation to determine the principles and optimum conditions of the ferrothermic extraction of copper from copper oxides.
Present methods of extracting metallic copper from its ores involve either a smelting or a leaching process. If the ore is sulfidic, a smelting process is usually used. This generally includes an initial roasting of the ore concentrate to remove excess sulfur, a smelting step to produce a matte of copper(I) sulfide, iron(II) sulfide, and siliceous slag, and a conversion step in which air is blown through the molten matte to obtain metallic copper and iron oxide, which combines with the slag.
The ferrothermic process proposed in this paper has several advantages over the smelting method. It would not require the addition of iron pyrite or other sulfur compounds to copper ores low in sulfur, a disadvantage of “matte” smelting that is compounded when these materials are not available in the vicinity of the smelter site. Similarly, the slagging of iron oxide through silica additions, as well as the matte conversion to molten copper, would become unnecessary.
The major processes of copper extraction from nonsulfidic ores involve leaching operations. Copper oxide ore, from which about 9 percent of the Nation’s copper production is obtained, is usually leached with dilute acids or ammoniacal ammonium carbonate, followed by electrolysis or cementation of the leach liquor to extract the copper. However, leaching methods are inefficient when copper is in the form of carbonates (malachite or azurite, both invariably associated with dolomite and limestone), silicates (dioptase), or mixed sulfide-oxide ores. Ores containing carbonates and silicates require calcining to copper oxides before the leaching process can be applied. Silicate ores dissolve slowly and are acid-consuming because of the formation of silica gel, which causes difficulties in the process. Mixed sulfide-oxide ores could probably be more effectively treated by methods similar to those recently applied to upgrade sulfide ores, in which the concentrate is heated and the copper oxidized in air using a fluidized-bed technique. Used in conjunction with such a conversion technique, the proposed metallothermic reduction should be very practical because the oxide would be at sufficiently high temperatures for the reaction to proceed.
In addition to those already mentioned, the disadvantages of leaching methods include the cost of the leaching agent and the problems of water pollution arising from their use, as well as the concomitant change in acidity. Leaching operations are invariably slow, whereas the proposed pyrometallurgical method is very rapid once the thermal requirements are achieved. The major subsidiary benefit to be expected from ferrothermic extraction of copper is in the potential use of ferrous scrap as a reductant, which could solve a solid-waste disposal problem, while offering a means for abating a water pollution problem.
Principles of Ferrothermic Reduction
Some of the possible reactions in the ferrothermic reduction of copper oxides are listed in table 1. These reactions are related to the Goldschmidt thermite process by which nobler metals can be obtained by heating their compounds with more electropositive elements. Iron appeared to be a suitable reductant for copper oxides because the amounts of free energy required for their reaction to yield various oxidation states of iron and metallic copper are relatively large and negative in the temperature range of 400° to 1,300° C. The standard free energy changes (ΔF°) for the reactions were calculated on the basis of thermochemical compilations made by Elliott and Gleiser, and these are also given in table 1.
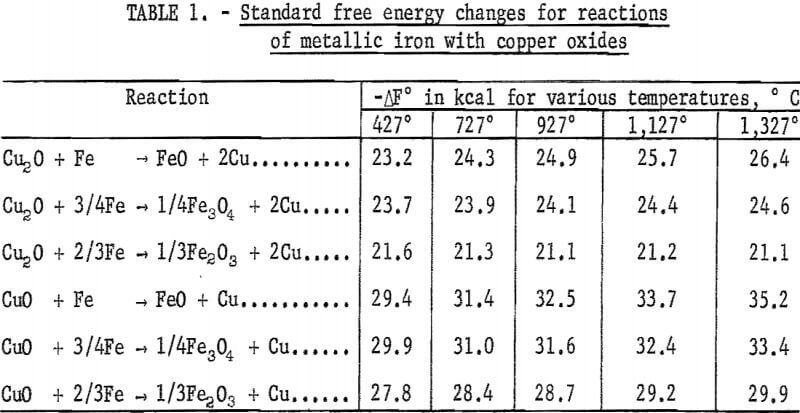
It is well established that thermodynamic considerations define only the lowest temperature at which a process is practicable and that higher temperatures are invariably required. The thermodynamic approach nevertheless enables one to predict the feasibility of a proposed process and thus may contribute to a considerable saving in its development cost. The graphical method of presentation, first proposed by Ellingham and Pourbaix proved to be extremely convenient because often it conveyed far more information at a glance than lengthy tabular presentations requiring tedious calculations in order to eliminate thermodynamically impossible reactions.
A compilation of standard free energy changes as a function of temperature for some pertinent reactions in the systems of iron-copper-carbon-oxygen-hydrogen is shown in figure 1. From this diagram it is immediately discernible
that metallic iron should be a better reductant of copper oxides than carbon below 700° C and that iron is almost as efficient a reductant of copper oxides as are hydrogen and carbon monoxide.
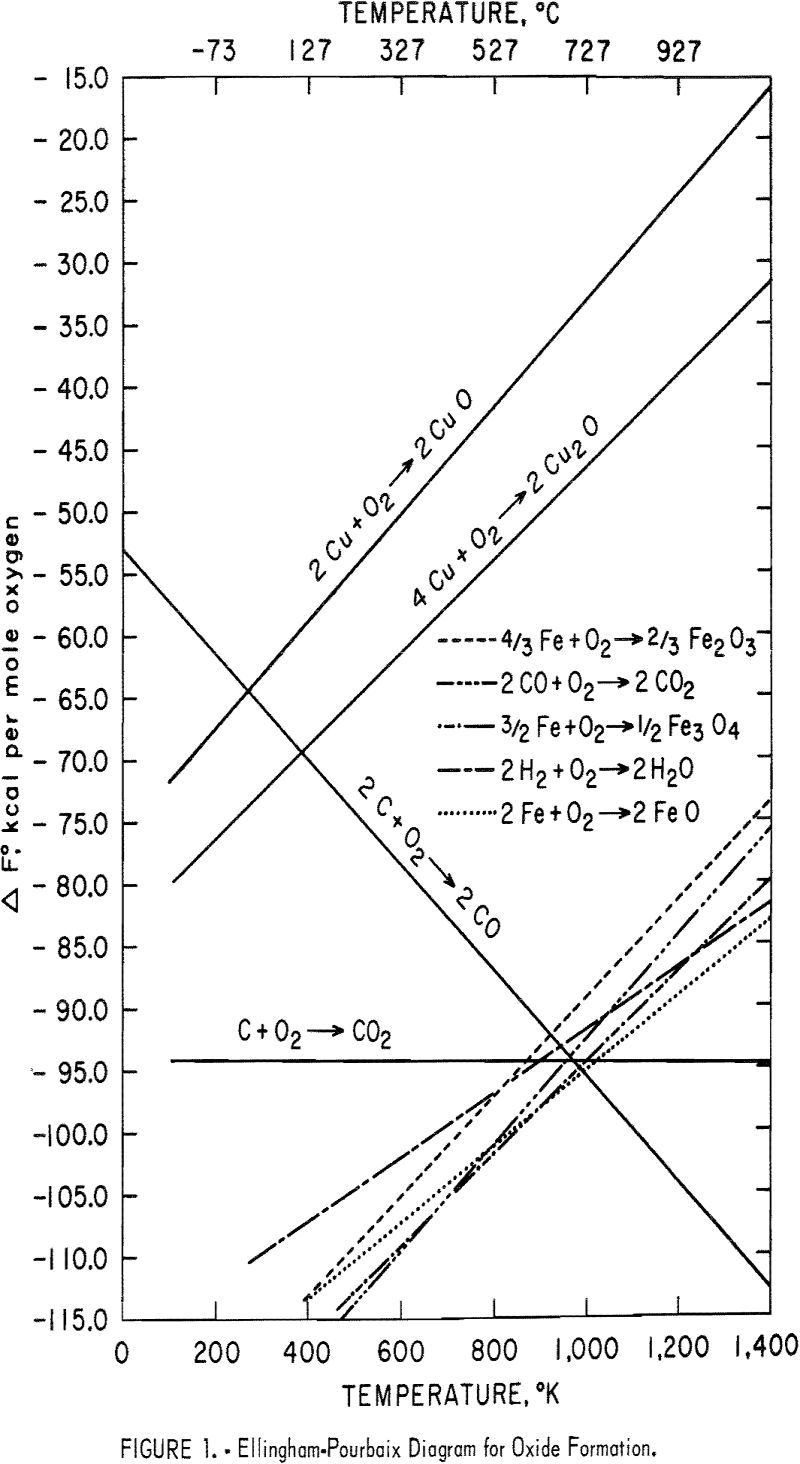
Ellingham-Pourbaix diagrams for the formation of sulfides of the foregoing elements are shown in figure 2. It is quite evident that carbon cannot reduce the sulfides directly because the standard free energy change for the formation of CS2 is less negative than that for copper or iron sulfides. It would also be anticipated that iron sulfides would oxidize more readily than copper sulfides above about 600° C because FeS is less stable than Cu2O (fig. 2), whereas FeO is more stable than Cu2O (fig. 1). Copper sulfides cannot, therefore, be reduced with metallic iron unless they are first completely roasted to the oxide.
The proposed method is essentially a solid-state cementation technique based primarily on the relative positions of iron and copper in the electromotive series of metals, This series arranges the metals in order of their decreasing tendency to lose electrons. Although the final separation of metallic copper from the accompanying iron oxides and gangue impurities is not dealt with in this investigation, the iron-copper phase relationships were considered in planning the experimental technique.
The iron-rich end of the iron-copper phase diagram, reconstructed from the cited references,is is shown in figure 3. If an iron-copper alloy is slowly cooled from the austenite (γ) region, it will form an iron-rich precipitate (α) as well as a copper-rich precipitate (ε). The solubility limits of copper in the α-phase and iron in the ε-phase decrease rapidly with falling temperature, reaching less than 0.2 percent at 600° C and probably even less at ordinary low temperatures. The maximum solubility of copper in iron (α- phase) is 2.1 percent, reached at 850° C. Also, the maximum solubility of iron in copper (ε-phase) is 3.5 percent, reached at 1,083° C, the melting point of copper. Because the solubility of copper in γ-iron is relatively large, care must be exercised in cooling the iron-copper mixtures from the hot reaction temperatures to room temperatures. Rapid quenching from the austenitic regions may result in formation of martensite in which the ferrite would be supersaturated with copper. Physical separability is theoretically easier from eutectic or eutectoidal mixtures and practically impossible from solid solutions, whether supersaturated or unsaturated. The experimental design in this research was planned in the light of the foregoing facts concerning the iron-copper phase diagram. Excessively high temperatures were not desirable because these would have put the system in the γ-space where copper has a high solubility in iron. Although operating at temperatures above 1,100° C would melt the copper and thus render it physically separable, this phase would contain about 4 percent iron. Separation of copper at lower temperatures would insure a virtually iron-free product. Finally, excessive amounts of iron would not be desirable; one should start with stoichiometric or nearly stoichiometric proportions of the reactants. The objective here was to reach a phase free of unreacted metallic iron so that the extracted
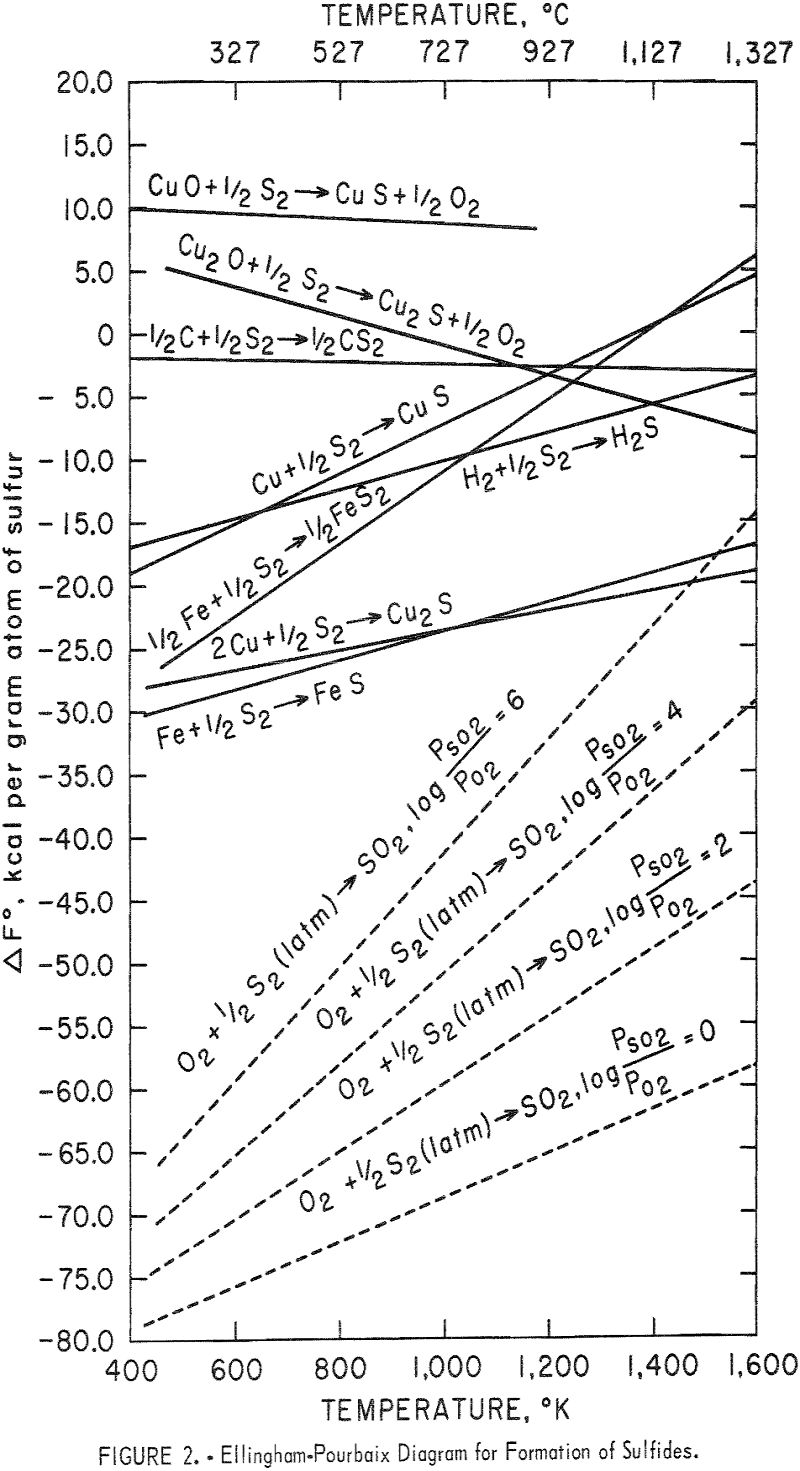
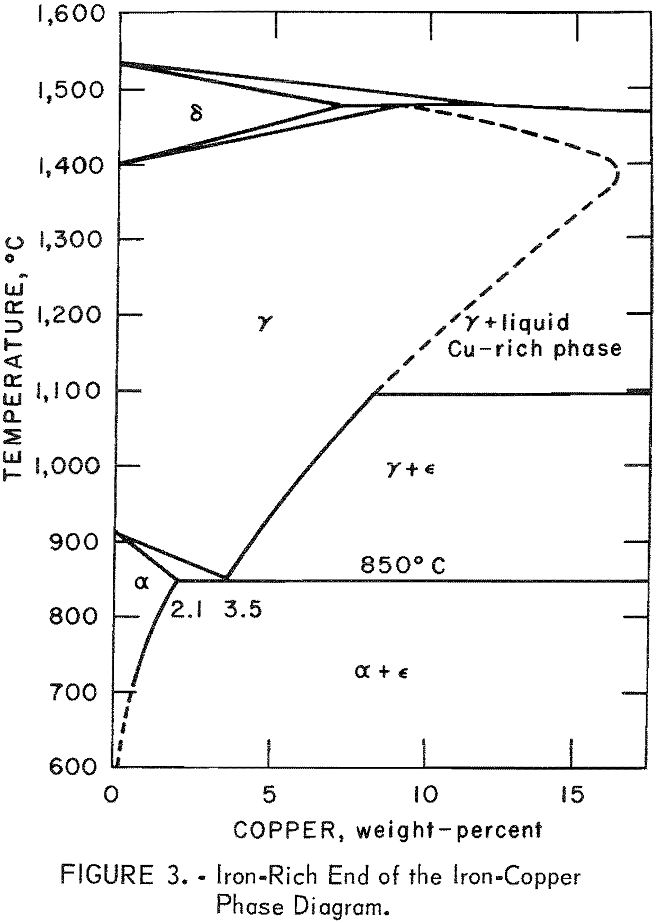
copper would not be contaminated. Ideally, one should get an iron oxide phase in which the copper has no solubility relationships. Experimentation was limited to the copper(I) oxide-iron system. The basic information developed should be helpful in adapting the process to real systems.
Experimental Procedures
The copper(I) oxide (Cu2O) used in these experiments was a Baker reagent grade powder. Three types of metallic iron were used as reductants:
- Minus 100-mesh iron powder (Plast-iron, grade A-210, manufactured by Plastic Metals Division of National U.S. Radiator Corporation).
- Tin can strips with dimensions of 5 by 23 cm and about 0.25 mm thick.
- Scrap iron from automobile bodies in the form of 2- by 3.5-cm strips about 1 mm thick.
Unless otherwise indicated, the experimental procedure was as follows:
The reactant proportions were adjusted to reach a copper-to-iron molar ratio of 2. This proportion corresponds to the first reaction, shown on table 1,
Cu2O + Fe → FeO + 2Cu…………………………………………………(1)
About 25 grams of the reactants were mixed for an hour in a V-blender and then placed in an alumina crucible. The charge was inserted in a vertical tube furnace, and after one-half hour of flushing with helium, the furnace was rapidly heated to the desired test temperature. The crucible was held at operating temperature for 3 hours and then slowly cooled. A helium flow of 0.14 liter per minute was maintained throughout the test. The reaction product was then removed, ground and analyzed for its total copper content.
The analysis was done by first dissolving the crucible discharge in a 1-to-1 mixture of HCl and HNO3, which was then dried and extracted with HClO4. Sodium thiosulfate solution was slowly added to the boiling solution in order to separate as black copper sulfide all the copper, which was associated with yellow free sulfur. The precipitated copper was redissolved in nitricperchloric acid mixture, which was then dried. This residue was dissolved in water, transforming it to the intense blue ammoniacal copper(II) ion, and then titrated with EDTA using murexide indicator.
Another analysis was performed on the reaction product to determine the sum of copper(O) plus copper(II). For this purpose, copper(I) was first extracted at -10° C from the solid mass by a mixture of ethyl alcohol and acid tin(II) chloride. The residue, containing metallic copper and copper(II), was then titrated for its total copper by the preceding method. The difference between the two analyses gave the value of unreduced copper(I) in the reacted mixture. The percentage removal of copper(I) was then calculated from the ratio of the two analyses multiplied into 100. Because chemical analysis for metallic copper in the presence of its oxidation states was not feasible, the percentage removal of copper(I) was considered the same as the percentage metallization or reduction of copper, based on the assumption that the amount of copper(II) would be zero or negligibly small because the furnace atmosphere was nonoxidizing or neutral. Thermodynamically, copper(I) is more stable than copper(II) in the temperature range studied, as is evident from a comparison of the free energy of formation of oxides and sulfides of copper, indicated in figures 1 and 2. Therefore, after starting with copper(I), it would be highly unlikely for copper(II) to form. This assumption was confirmed when the chemical analysis of a heated Cu2O blank indicated that the [Cu(O) + Cu(II)] content was less than 2 percent.
Results and Discussion
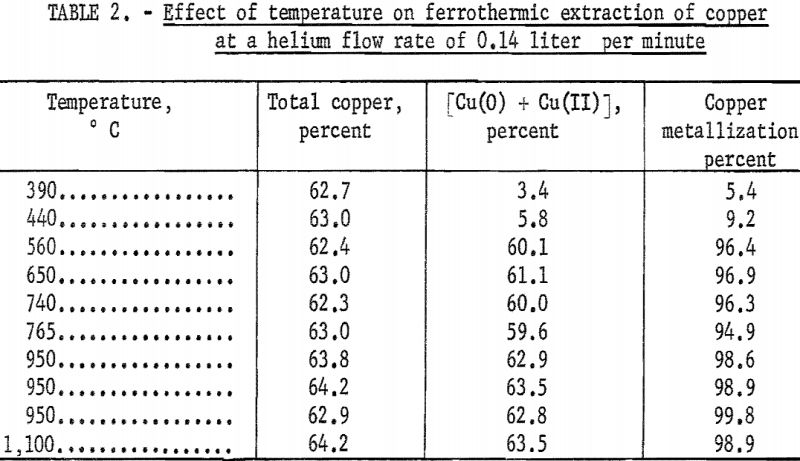
Effect of Temperature
The effect of temperature on the ferrothermic reduction of copper(I) oxide was investigated in the range 390° to 1,100° C. Experimental results of the percentage of copper metallization within 3 hours at 8 temperatures and with 2-to-1 molar ratios of copper to iron powder in a helium atmosphere are given in table 2. The variation of the percentage of copper metallization with the reaction temperature is shown in figure 4. It was found that over 95 percent of the copper was extracted by iron powder at 560° C. Above that temperature no significant increase of copper metallization was achieved. It must be recalled that the 3-hour copper metallization does not necessarily give a measure of the reaction speed. Instantaneous kinetic curves relating the percentage of copper metallization to time are planned in the future, and these should give a true assessment of the temperature effect.
Effect of Reactant Proportions
The effect of varying reactant proportions was studied at 765° C. Three different proportions of reactants were used, corresponding to molar ratios of copper to iron of 1, 2, and 8/3, As mentioned previously, a molar ratio of 2 corresponds to the wustite-forming reaction (reaction 1, table 1). Likewise, a molar ratio of 8/3 should correspond to the magnetite-forming reaction (reaction 2)
4Cu2O + 3Fe → Fe3O4 + 8Cu………………………………………………………………(2)
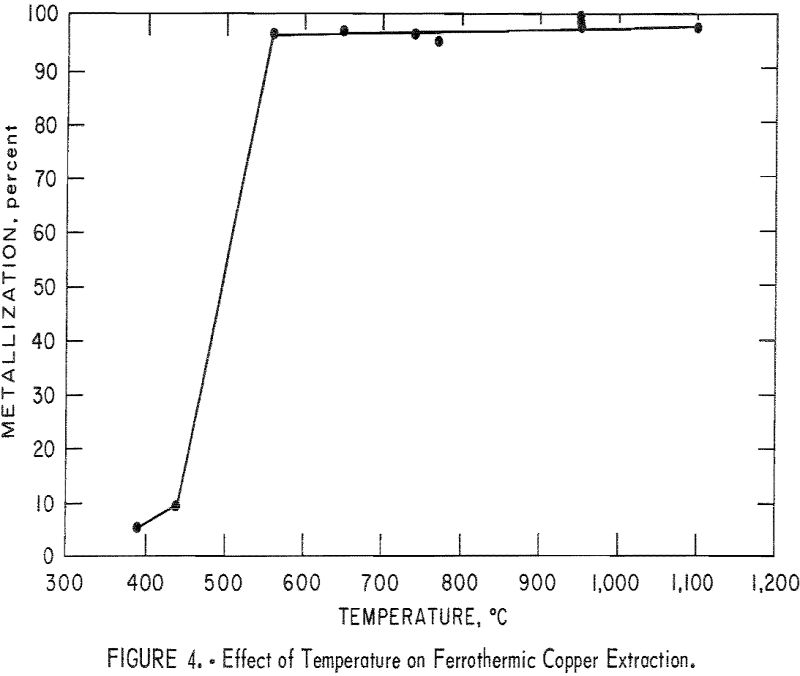
Although molar ratios of less than 2 are still consistent with reaction 1, they connote an abundance of the reductant beyond the stoichiometric requirement. The results are given in figure 5, which shows the percent of copper metallization plotted against the molar ratio of the reactant. As expected, increased reduction was obtained as the iron content was increased. The percentage of copper metallization appeared to decrease drastically at molar ratios of more than 2. Although the free energy change for the magnetite- forming reaction is slightly more positive than the wustite-forming reaction, this significant effect of molar ratio appears to be related to the available surface area of the iron base with which the copper oxide can react.
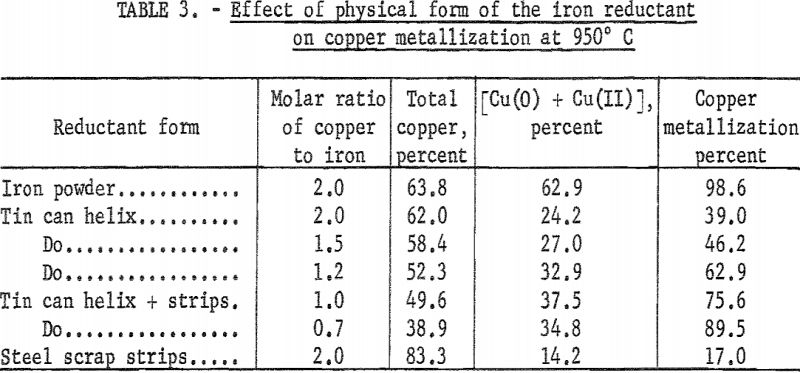
Effect of Physical Form of the Reductant
The effect of three forms of metallic iron reductant was determined at 950° C. In the first experiment, iron powder was blended with Cu2O as described previously. In some experiments, the reductant consisted of a helix made from the sides of tin cans. In other tests, the tin cans were sheared into 1/8-inch-wide strips which were embedded in the helix with the gaps filled with Cu2O. The helix was inserted into an alumina crucible and surrounded on all sides with Cu2O. The weights of the required oxide powder and the reductant were determined. The molar ratio in each test is given in the second column of table 3. In one experiment, four metallic iron strips cut from scrap automobile bodies were used as the reductant. The strips were embedded in Cu2O contained in an alumina crucible while maintaining a molar ratio of 2. Visual examination of the reaction product revealed some copper cladding on the iron strips and the tin cans.
The results of these runs are given in table 3. It is interesting to note that the percentage of metallization obtained with iron from tin cans was higher than that obtained with steel scrap and that it varied with the mode of preparation of the charge. On the other hand, the percentage of metallization achieved with steel scrap was considerably less than that obtained with iron powder. It must be remembered that the desired reaction depends on the oxidizability of iron and that steel scraps are usually manufactured to withstand corrosion and oxidation. Methods now being developed by the Bureau of Mines to enhance the oxidizability of steel scrap could possibly be invoked here to increase the copper metallization yields. The present investigations did not delve into this aspect of the research.
Effect of Gaseous Environment
The effect of varying the atmosphere over the reaction mixture was studied with two different systems. The first system was composed of Cu2O and iron powder in a molar ratio of copper to iron of 8/3; the second system was composed of Cu2O and steel scrap in a molar ratio of copper to iron of 2. Four experiments were run on the first system at a constant temperature of 760° C and under nearly identical conditions, except for the atmosphere surrounding the reaction mixture. The first experiment was performed in a flowing helium atmosphere of 0.14 liter per minute. In the second experiment, a static helium atmosphere was employed. The reaction chamber was flushed out with helium prior to heating. The helium flow was then turned off, and the reaction chamber was heated to provide an essentially static helium atmosphere. In the third experiment, the atmosphere was changed to a static helium atmosphere containing water vapor. This gas composition was accomplished by bubbling helium through a water bath maintained at 25° C. The gas mixture was passed through the reaction chamber for 30 minutes prior to heating. The gas was then turned off, and the reaction chamber was heated to give a static atmosphere composed of helium and about 3 percent H2O. For the fourth experiment, a static atmosphere composed of helium and 1 percent CO2 was used. To obtain this composition, the reaction chamber was initially flushed with helium, then evacuated. Helium was then admitted to the reaction chamber until the manometer read 99 percent of atmospheric pressure. The remaining 1 percent was filled with CO2 to give the desired gaseous environment.
The results of these experiments, as well as the analyses of the reaction products, are given in table 4. It was suspected that the addition of H2O and CO2 to the helium atmosphere might enhance the reaction by converting it from the usually sluggish solid-solid type to a cyclic gas-solid type, shown by reaction 3, and similar to the conditions that prevail in magnetic roasting of iron ore with steel scrap.
The results obtained indicate that a static atmosphere is generally preferable to a flowing atmosphere. Although small amounts of water vapor appear to exert a beneficial effect on the ferrothermic extraction of copper, traces of carbon dioxide have a slight adverse effect.
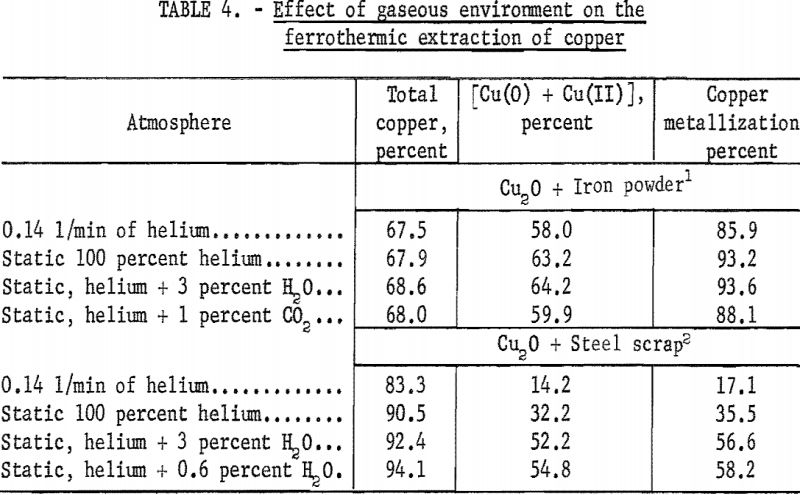
The relative effect of the gaseous environment was essentially the same with both iron powder and steel scrap reductants and appears to be independent of the reaction temperature. The data in table 4 show clearly that a static helium atmosphere with a very small amount of water vapor is the most desirable condition for promoting the interaction between Cu2O and steel scrap. With iron powder, this conclusion was not clearly evident, probably owing to the fact that the reduction values were already reaching their upper saturation values. Replacement of helium with nitrogen gave essentially the same results reported in table 4. Helium was preferred to nitrogen in this type of basic investigation because it could be obtained in a much purer form and did not require passage over heated copper foil to remove oxygen.
Summary and Conclusion
The results of this investigation indicate that the most favorable conditions for reacting iron with Cu2O are temperatures in excess of 560° C and a static helium or nitrogen atmosphere containing approximately 0.6 percent water vapor. Increasing the molar ratio of copper to iron from 1 to 3 resulted in a decrease in copper metallization. The reactivity of Cu2O with tin cans and heavier steel scrap was variable and less than that with powdered iron. This ferrothermic process seems to involve high-temperature, solid-state cementation of copper on an iron base.
The practical application of this process on a large scale would involve continuous feeding of iron scrap and oxidic copper ore concentrate to a rotary kiln heated to the optimum reaction temperature and containing an inert atmosphere with small amounts of H2O. Sulfidic copper concentrates could be reduced with metallic iron following their complete oxidative roasting, because the oxide would then be at a sufficiently high temperature for the reaction to proceed.
