Table of Contents
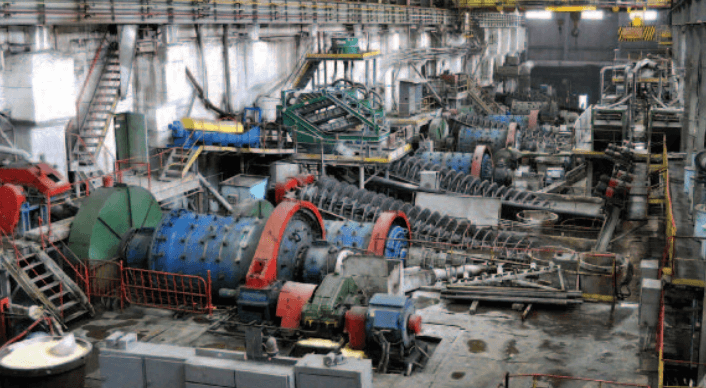
The present great demand for tungsten due to world conditions has resulted in an increase of tungsten ore dressing problems for our metallurgists. The flowsheet study shown here was recently developed for treatment of a very complex tungsten-gold ore.
The flowsheet study previously shown for tungsten was devised for a simple tungsten ore free of other heavy minerals. The first consideration of any tungsten ore is to recover the mineral as coarse as possible and avoid sliming and overgrinding by stage crushing and grinding. This is necessary because of the friable nature of all tungsten minerals. This fact was kept in mind for the study shown here, but the problem was complicated due to the occurrence of iron sulphides and free gold with Scheelite.
Straight gravity concentration of this ore, using the flowsheet shown, would result in a mixed gold-tungsten concentrate containing much sulphide which is not desirable for marketing. Consequently, it is evident that other concentration processes must be incorporated in the flowsheet to produce separate marketable concentrates of tungsten and gold. There are numerous possibilities that could be applicable to this problem, but the described flowsheet offered the greatest degree of flexibility.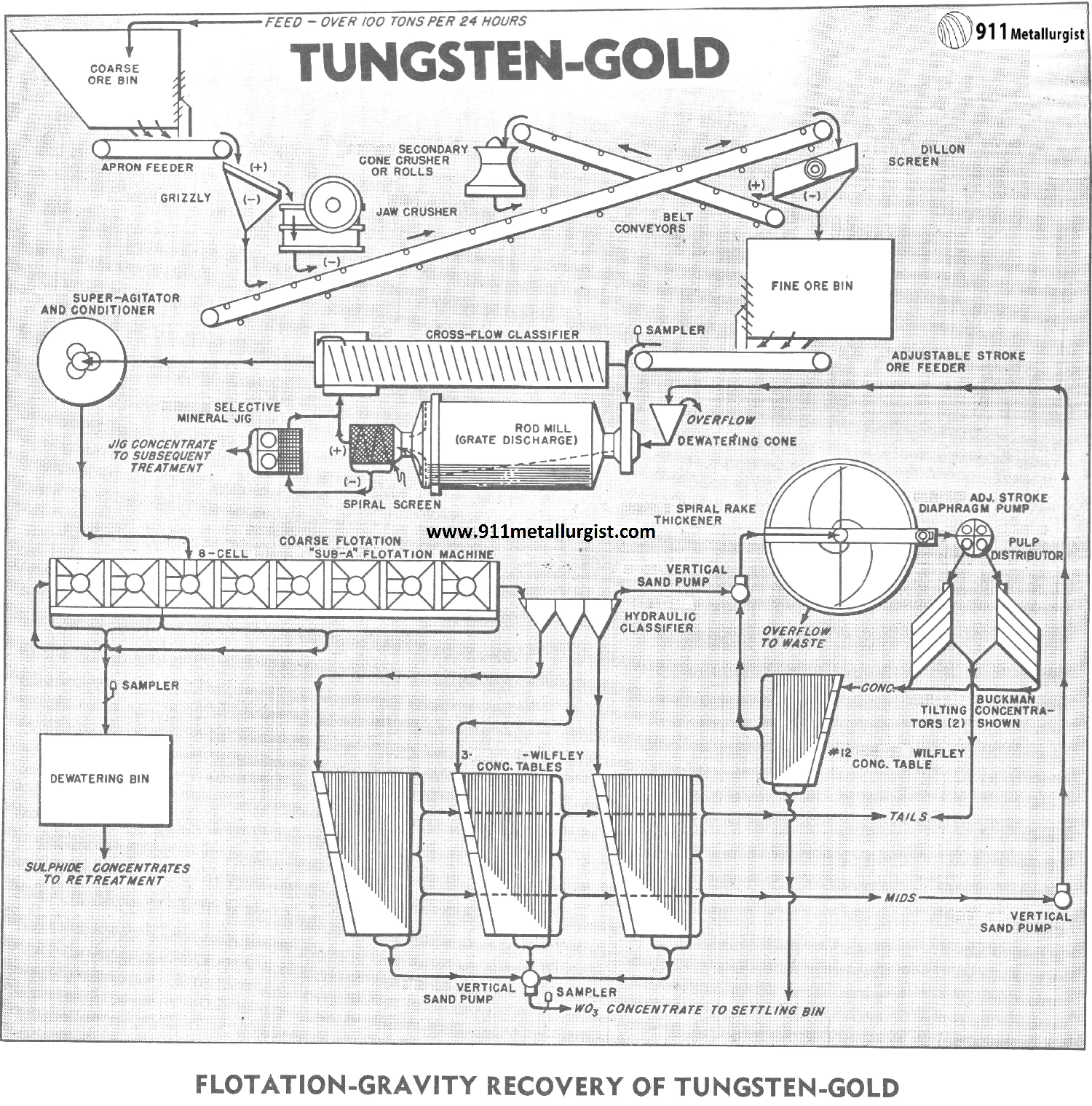
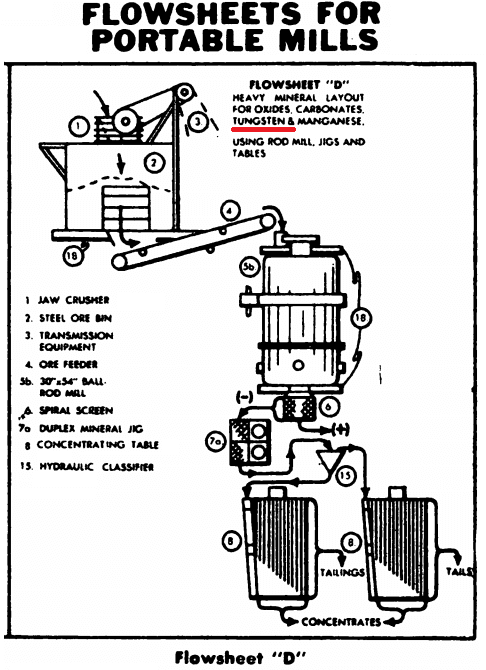
THE Tungsten Rich Wolframite Ore FLOWSHEET
Tungsten Rock Crushing Circuit
The crushing section is designed to remove the undersize between each crushing step and thus prevent excessive production of fines. Primary crushing is accomplished by the Forced Feed jaw Crusher which discharges to a conveyor and then to a Vibrating Screen. The screen oversize is crushed in a secondary cone crusher and the crushed product returned to the vibrating screen.

Crushing Rolls (with large diameter shells) could be employed in place of the cone crusher and in some cases, would probably be preferable. Note the ingenious features of this crushing circuit whereby the jaw crusher and cone crusher products are conveyed to the vibrating screen on common belt conveyor. This arrangement requires one less conveyor belt than old- style crushing flowsheets.
The vibrating screen undersize passes to a fine ore bin for storage prior to grinding. A possibility not used in this flowsheet but sometimes advisable, is to remove the approximate minus 10 mesh fraction from the crushed ore and treat this product on a Mineral Jig prior to grinding.
Tungsten Grinding Circuit
The crushed ore from the fine ore bin is ground in a Rod Mill to free the minerals with as little sliming as possible. The rod mill is of the special grate-discharge type which is extensively used for tungsten and many non-metallic ores where overgrinding is a serious factor.
The free sulphides, gold and tungsten, are removed in the grinding circuit by means of the Mineral Jig. This jig is selective in its action and is particularly suited for the recovery of fine mineral as well as coarser particles in a high grade product. For simplicity, retreatment of the jig concentrate is not shown, but this includes regrinding with the possibility of amalgamation, flotation, and cyanidation to produce gold in either concentrate or bullion form and a high grade Scheelite concentrate.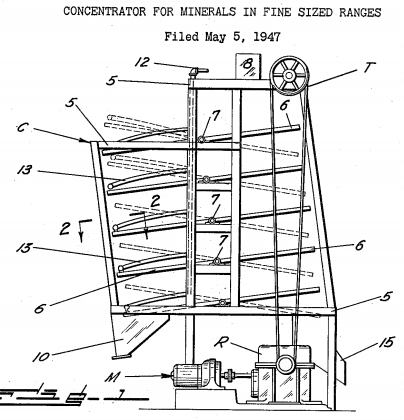
In some cases the treatment of the jig concentrate may not require additional grinding but can be subjected to roasting followed by magnetic separation to remove pyrite. Magnetic separation may also be employed to concentrate Wolframite or other magnetic tungsten minerals, such as Ferberite and Huebnerite. Scheelite, a calcium tungstate, is non-magnetic.
Tungsten Flotation Circuit
The classifier overflow passes by gravity to a Super-Agitator and Conditioner prior to “Sub-A” Flotation. The object of the flotation section is to remove the remaining fine gold and sulphides before table concentration for the recovery of tungsten. A rougher concentrate is shown, but this can be cleaned, and a high grade concentrate produced in the same flotation machine, provided that a “Sub-A” Flotation Machine is used.
Shaker Table Circuit
The flotation tailing is hydraulically or mechanically classified and tabled to produce a finished high grade tungsten concentrate, a middling product, and a final tailing. The table middling is pumped back to the rod mill for regrinding after being dewatered in a spitz-type dewaterer.
Buckman Tilting Concentrator to recovery Wolframite
The overflow slime product from the hydraulic classifier is thickened in a Spiral Rake Thickener and treated on Buckman Tilting Concentrators to recover the extremely fine tungsten mineral. These Tilting Concentrators play a most important part in recovering the mineral in the fine size range. The concentrate from the Tilting Concentrators is cleaned on a half-size table to produce a final tungsten concentrate. It is considered a “must” to use these Buckman Tilting Concentrators for recovering fine, heavy minerals at low cost and without the need of specially skilled operators.
Mill results are accurately controlled by means of Ore and Pulp Samplers at strategic points throughout the circuit.
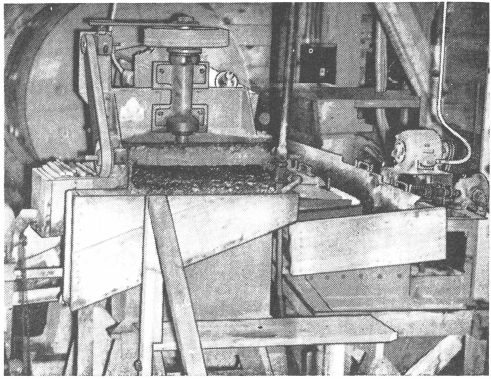
When treating tungsten, consider using a Mineral Jig (Pulsating Selector) ahead of the Ball Mill. Friable tungsten minerals may be concentrated at this point in the circuit avoiding possible slime losses.
Summarizing the Tungsten Recovery & Wolframite Beneficiation
The flowsheet shown here, wherein a mineral is recovered as soon as it is freed, utilizes the basic concentration principles for tungsten but is complicated by the complex nature of the ore. To insure success, an ore test in the Ore Testing Laboratory is always recommended. While the flowsheet shown is for a specific ore, it is sufficiently flexible to be adapted to other ores with slight modifications.
 |
 |
https://almonty.com/tungsten/history/
https://www.911metallurgist.com/tungsten-processing
Beneficiation of Microsize Wolframite by Flocculation and Gravity Separation
Materials, Wolframite was obtained from Jiangxi, physically purified and ground in porcelain mill, getting -10 µm microsize by a sedimentation. The sample was assayed for 71.57% WO2. The quartz sample (-19 µm) was purchased from changsha mineral preparation Co, contains 99.4% SiO2 and 0.6% impurity. Some was acid-treated and washed with distilled water repeatedly until no any traces of Cl- ion being detected.
The synthetic polymers of nonionic, partially hydrolyzed (30%) and sulphated (40%) polyacrylamide (PAN, HPAN, PANS) were used as floccuants in SFGS process. Collectors of styryl phesphonic and benzyl arsonic acid were also accepted in the test.
Polymer Flocculation, Sedimentation was used in a similar way as described by Sresty and Somasundaran to measure flocculation response to single mineral in the suspensions. The flocculation efficiency Ef was referred to the work J.Chen, et al (1988).
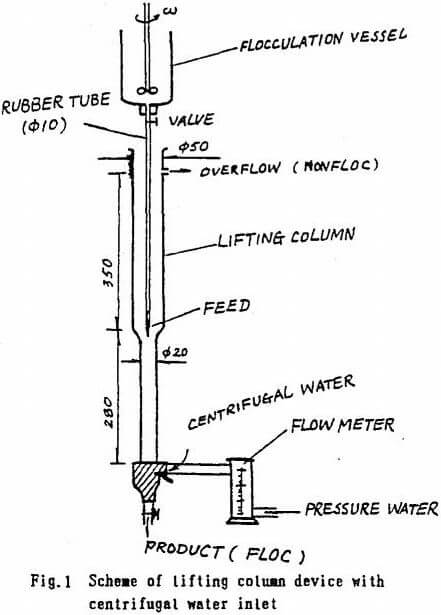
Separatory units for mixtures of EVT (type XYZ 400 X500) made in Xichang Factory 102, China) and LC (∅ 50 +20, designed by authors with centrifugal water inlet, as in Fig.1) were tested.
The cross-belt separator XYHL 700 x 1200 was used in treatment of natural ores slimes flocculated by polymers.
Flocculation & Dispersion Behaviors of Single Minereral
The flocculation respone of polymers, CF, (PAMS : HPAM = 1 : 1), CF, (PAMS : PAM = 1 : 1) and PAMS, is given in Fig.2 It can be seen that CF, is the best choice among these polymers. The reason that co-reagents promote Ef is association between polymers, crystal defections on wolframite. Polymers look inactive with quartz.
The PH of pulp can regulate electrokinetic eviornment to enhance the performance of polymers to greater extent. CF, is effective in PH 3 – 8.5, but HPAM as below PH 7. It illustrats that co-reagent can extend PH ranges. Theoritically, the PZC of wolframite is 2.6-2.8, and PZC (quartz) is 1.8-2.0. If media PH>PZC, the mineral is negatively charged. The potential curves similar to “horse saddle” are shown in Fig.3 with Electropenetrater.
In presence of anionic CF, the negative value of potential on wolframite is considerably increased. It shows that anionic CF, still strongely flocculates wo-
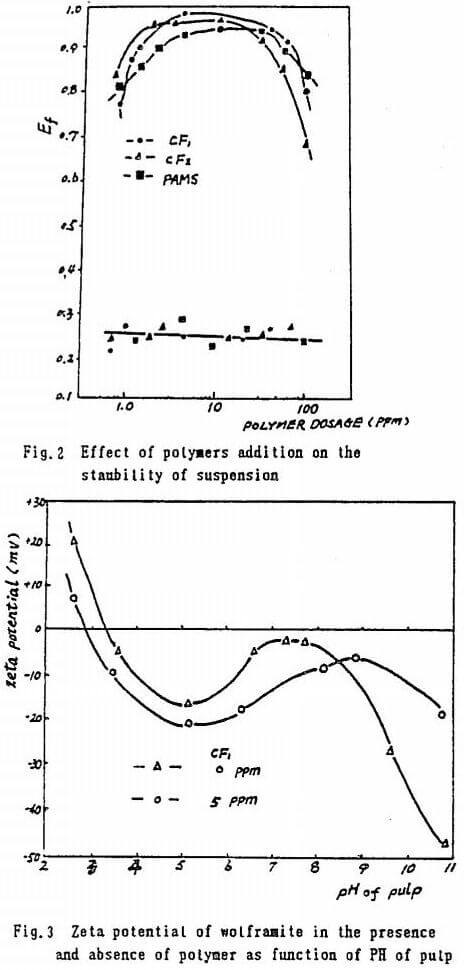
lframite charged negatively. Apparently, CF, is of predominantly chemosorption than electrostatic function on the mineral.
Measurements of infrared spectra provide the evidence, implying actively anionic group of the polymer forms chemical bonding with Mn(II) in the crystal structure of wolframite. See Fig.4.
The adquate dispersion before flocculation affects flocculation response. The test results show that the addition of Na2SiF6, CMC can keep higher level of Ef in dosage of 5—80 mg/L, but (NaPO3)4 greatly depresses wolframite over 6mg/L dosage. Water glass added in suspension must be precisely controlled, or wolframite is generally depressed in alkaline medium.
The density of pulp in 1~8% solid by wt. is suitable, and Ef is graduately reduced above 8%. Pulp density affects both yield and separatory effeciency E, expressed as Douglas.
For polymer-flocculated systems, agitation is necessary to provide adquate mixing of polymer with the suspension, and to improve particle-polymers and particle-particle collisions leading to destabilization and floc growth. Excess agitation has usually broken up flocs. It is found that good Ef occurs in the stirring rate 900-1200 r.p.m. over 3 min.
Behaviors of Flocs on Gravity Units
Floc is different from common mineral particles. It is characterized as follow, coarser size distribution, less in shape coefficient and density, easier in degradation. Ideal case should be such that valuable mineral is selectively flocculated completely, but associated gangue is adequately dispersed in studied systems. Unfortunately, the gangues are partially flocculated due to poor selectivity of polymers.
The reason with higher E, by SFGS process for natural minerals or mixture is that, interaction between gangue and modified polymer is mostly physical adsorption, thus formed flocs in suspension are larger in porosity and weaker in its strength. However, valuable minerals, relatively speaking, is stronger in floc structure and less porosity due to chemosorption between the mineral and polymers. This will provide advantageous conditions for separating flocs. Consequently, the difference of relative density between two kinds of minerals is greatly enlarged before and after flocculation. Under weak shearing produced by the a-tion of flow or mechanical forces, Bagnold’s repulsive forces facilite separatory results of flocs.