Table of Contents
- Description of McDermitt Clays
- Extraction Studies
- Acid Baking Water Leaching
- Roast Tests
- Alkaline Roast Water Leach
- Sulfate Roast Water Leach
- Chloride Roast Water Leach
- Multiple Reagent Roast Water Leach
- Potassium Chloride-Calcium Carbonate Roast-Water Leach
- Potassium Chloride-Calcium Sulfate Roast-Water Leach
- Roast Leach Treatment Summary
- Recommendations about Roast Leach Treatment
The Federal Bureau of Mines investigated extraction of lithium from non-conventional resources in keeping with its goal of maximizing minerals and metals recovery from domestic resources in order to assure an adequate supply of minerals to meet national economic and strategic needs. This report describes the results of bench-scale studies on the processing characteristics of two lithium-containing clays from the McDermitt Caldera near the Nevada-Oregon border. This information is presented to provide a data base for further investigations.
The national economic and strategic needs for lithium in 1990 are projected to be 10 times the present production rate. The potential energy-related uses of lithium are expected to rise dramatically in the decades following 1990. Lithium has the greatest potential of any  element for the manufacture of lightweight batteries for electric automobiles. Even a moderate production of electric automobiles could increase the lithium demand significantly. Longer range needs for the development of thermonuclear power will place even greater demands on lithium resources. Figure 1 shows a combination of projected lithium requirements for the years 1980 to 2020. As shown in this figure, lithium demands for the present uses (ceramics lubricants, aluminum reduction, refrigeration, and air purification) are projected to increase by 5 pct per year. Lithium-aluminum/iron-sulfide batteries could be introduced as early as 1985. The projected lithium requirements are shown for these batteries, which would be used for both utility energy storage and electric vehicles. A 10-year battery life and 90-pct recycle of lithium from used batteries was assumed, The potential lithium demands for controlled thermonuclear reactors are projected to cover the lithium requirements for reactors providing 2,070 billion watts electricity (GWe) in the year 2010 and 2,525 GWe in 2020, Figure 1 shows the demands for the reactor concept in which the liquid lithium blanket performs the simultaneous functions of tritium breeding and reactor cooling. This blanket-type system requires a large lithium inventory but has a small fractional burnup rate of lithium-6.
element for the manufacture of lightweight batteries for electric automobiles. Even a moderate production of electric automobiles could increase the lithium demand significantly. Longer range needs for the development of thermonuclear power will place even greater demands on lithium resources. Figure 1 shows a combination of projected lithium requirements for the years 1980 to 2020. As shown in this figure, lithium demands for the present uses (ceramics lubricants, aluminum reduction, refrigeration, and air purification) are projected to increase by 5 pct per year. Lithium-aluminum/iron-sulfide batteries could be introduced as early as 1985. The projected lithium requirements are shown for these batteries, which would be used for both utility energy storage and electric vehicles. A 10-year battery life and 90-pct recycle of lithium from used batteries was assumed, The potential lithium demands for controlled thermonuclear reactors are projected to cover the lithium requirements for reactors providing 2,070 billion watts electricity (GWe) in the year 2010 and 2,525 GWe in 2020, Figure 1 shows the demands for the reactor concept in which the liquid lithium blanket performs the simultaneous functions of tritium breeding and reactor cooling. This blanket-type system requires a large lithium inventory but has a small fractional burnup rate of lithium-6.
At present, the United States is meeting all of its lithium needs by production from conventional spodumene deposits in North Carolina and from one unique underground brine deposit in Nevada. Production from these sources can meet all short-range requirements, but the longer range resource status is not well defined. The probable reserves of lithium in domestic pegmatite ore are 65,000 metric tons. Although lithium-containing brines also are a potential resource, the major reservoir of these brines is in Chile. In the United States, both identified and hypothetical resources of lithium in pegmatites and brines combined probably total at most 3.5 million metric tons. Therefore new deposits of lithium in nonconventional resources must be identified as an aid to future planning.
In 1974, the Lithium Resource Appraisal Group of the U.S. Geological Survey, Denver, Colo., was formed to evaluate domestic lithium resources. Their preliminary work disclosed that various types of clays in the Western States contain quantities of lithium sufficient to constitute a major new lithium resource. The Bureau of Mines lithium extraction program was conducted in cooperation with the U.S. Geological Survey. Clay samples were supplied by the Lithium Resource Appraisal Group, and experimental results were reviewed with the group’s personnel.
The clays were first subjected to mineralogical studies , which indicated that it would not be practical to upgrade them by conventional physical beneficiation techniques. For this reason, the Bureau investigations were conducted primarily on the raw clay samples as received.
This report presents the results of extraction tests conducted on two samples of hectorite-type clay. Suggestions for future investigations are also offered.
Description of McDermitt Clays
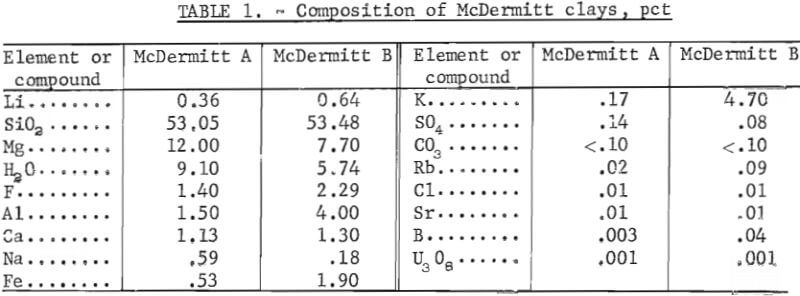
Lithium extraction tests were conducted on hectorite-type clays from an extensive deposit near the Oregon-Nevada border. This area, designated as the McDermitt Caldera, is described by Glanzman, Rytuba, and McCarthy. McDermitt clays may constitute a potential lithium resource because they contain up to 0.6 pct Li; this concentration is well above the average lithium concentration in clays (0.006 pct). The McDermitt Caldera is about 26 miles in diameter and is a result of volcanic activity, subsidence, and sedimentation. The lithium content probably is derived from volcanic material and was incorporated into the clay beds during alteration. Lithium is enriched in clays that are found in a rough crescent shape extending from the northeastern corner to the southwestern section of the caldera.
Nine clay samples from the McDermitt Caldera were supplied by the Lithium Resource Appraisal Group of the U.S. Geological Survey. Six of the samples were from the northern section of the caldera. Clays in this area contain as much as 0.36 pct Li and are associated with clinoptilolite and feldspar. Three of the samples were from the southwestern section of the the caldera. These clay contain up to 0.64 pct Li and are associated with analcime and potassium feldspar. Lithium extraction tests were run on one sample that was typical of the lower grade clays from the northern section and on one sample that was typical of the southwestern section of the caldera.
X-ray diffraction studies of the samples showed that both are montmorillonite-type clays. The principal difference in the clays is grain size. Figures 2 and 3 are scanning electron photomicrographs of the samples at X 7,000 magnification. The sample that contains 0.36 pct Li (fig. 2) shows no discrete grains at this magnification. In this report, this fine gram sample will be referred to as McDermitt A. In figure 3, the sample that contains 0.64 pct Li shows discrete, flakelike grains. This coarser grain sample will be referred to as McDermitt B.


The two samples were stage-ground to minus 10 mesh. The ground samples were dry-screened into six fractions from minus 10 plus 20 mesh to minus 200 mesh. No size fraction contained a higher percentage of lithium than the head sample. The entire minus 10 mesh sample was ground to pass 100 mesh to provide feed material for the extraction tests. Compositions of McDermitt A and McDermitt B are in table 1.
Extraction Studies
A wide variety of approaches is possible for extracting lithium from clays. The choice of which approach to follow depends upon the nature of the specific raw material being considered. Although many lithium extraction processes have been reported, most of the processes have been developed for pegmatite raw materials and may not be effective for extracting lithium from clay feed material. Previous Bureau of Mines studies have investigated lime-gypsum roast and chloride roast for lithium extraction from spodumene and amblygonite. The techniques chosen for extracting lithium from clays in this investigation were (1) water disaggregation; (2) hydrothermal treatment; (3) acid leaching; (4) acid baking-water leaching; (5) alkaline roasting-water leaching; (6) sulfate roasting-water leaching; (7) chloride roasting-water leaching; and (8) multiple-reagent roasting-water leaching.
Leach Tests
One of the simplest approaches toward removal of any constituent from low-grade raw materials is direct leaching to solubilize desired elements. The following leaching techniques were investigated: (1) water disaggregation; (2) hydrothermal treatment; (3) acid leaching; and (4) acid baking-water leaching.
Water Disaggregation
The McDermitt clays were slurried with water to (1) determine whether these samples would disaggregate and form a lithium-rich fraction, and (2) determine the amount of lithium that was water-soluble. Twenty-five-gram samples of the clays were slurried with 225 ml of water and agitated for 1 hour. The slurry prepared with McDermitt A showed slight gelation, but the McDermitt B slurry did not form a gel. The mixtures were screened; analyses of the products showed that these clays did not disaggregate to form a lithium- rich fraction and that less than 1 pet of the lithium in each sample was soluble in water.
Hydrothermal Treatment
Hydrothermal tests were conducted to determine if lithium in the clay structure can be transferred to water solutions under relatively mild conditions of heat and pressure. In each test, 40 grams of clay was mixed with 360 ml of water and heated to 200° C in an autoclave for 1 hour; the steam pressure was 225 pounds per square inch. Analyses of the solids and water solutions obtained showed that less than 1 pct of the lithium was solubilized by this treatment.
Acid Leaching
Both McDermitt clays were leached with sulfuric acid at pH 1 to determine if the contained lithium was acid soluble. Twenty-five grams of each clay was added to 225 ml of pH 1 sulfuric acid, and the slurries were stirred for 3 hours. Sulfuric acid was added every 10 minutes to maintain the solutions at pH 1. The amount of acid required to achieve the pH 1 terminal acidity was designated as the “standard” acid addition for that material. The next tests were conducted with two and three times the “standard” acid addition to determine whether the amount of lithium extracted was directly proportional to the amount of acid used. Results of these direct acid leaching tests are shown in table 2.

This table shows that lithium can be extracted from McDermitt A by direct acid leaching, but the required acid addition is large. The acid required to solubilize 89 pct of the lithium in this clay is equivalent to 1,125 pounds of 98-pct acid per ton of clay. In contrast, only 1 pct of the lithium in McDermitt B was acid soluble at 1 pH.
A number of tests were also made to determine if preleach heat treatment of the clays would alter the acid leaching characteristics. These tests were made because information from the U.S. Geological Survey suggested that heating the two clays could change their crystal structure. The clays
were heat-treated at temperatures from 400° to 1,000° C, and the calcines were acid leached at pH 1. The amount of lithium leached from the heat-treated samples was not significantly higher than the amount leached from the raw clays.
Acid Baking Water Leaching
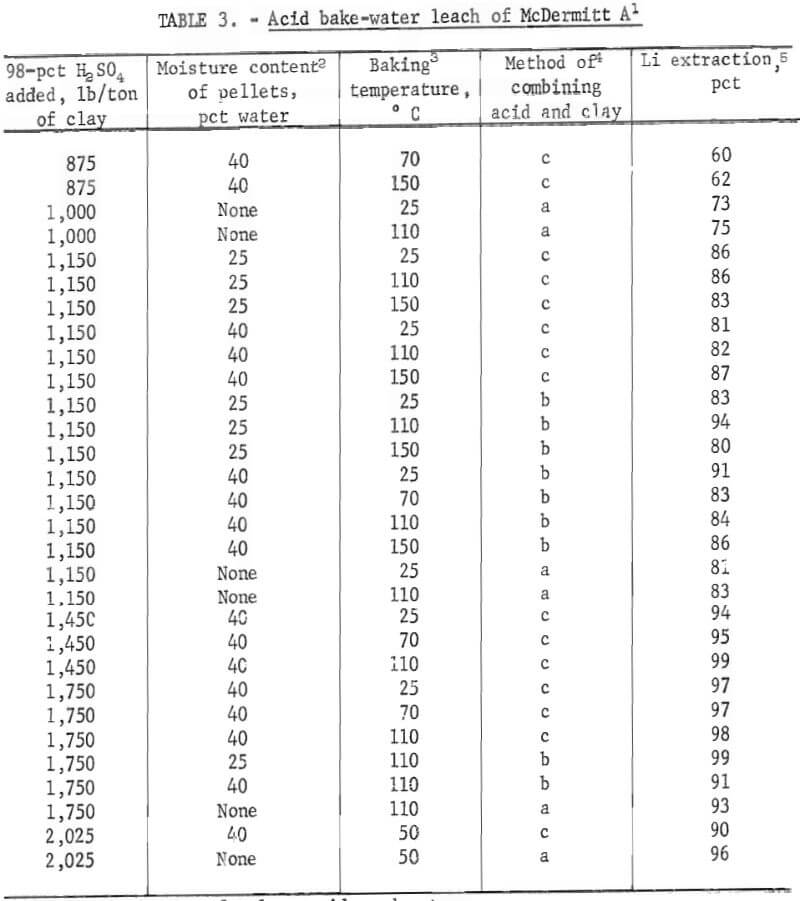
Acid baking-water leaching tests were conducted on McDermitt A but not on McDermitt B, because direct acid leaching had shown that McDermitt B did not respond to acid treatment. The goal of the acid bake-water leach tests was to determine if the high acid concentrations possible with this technique would improve the effectiveness of the acid. In this technique, concentrated sulfuric acid was added directly to the clay and not to a water slurry of the clay. The acid-clay mixture was baked, and the baked product was leached with water to extract soluble lithium sulfate. The clay raw material was dry or in the form of damp pellets. To prepare the damp pellets, dry clay was tumbled in a small rotary pelletizer and sprayed with water until pellets formed. Pellets formed in this way retained their shape and strength when treated with concentrated sulfuric acid. Pellets were also formed by adding both concentrated acid and water to the dry clay as it tumbled in the pelletizer. With either preparation technique, when the concentrated acid was added, the heat of dilution was released within the clay-water mixture and heated the mass of pellets. Tests showed that when concentrated acid was added to damp pellets of McDermitt A sufficient heat was released to heat the mass to 80°C.
The clay-acid-water pellets were baked in an open vessel for 1 hour at temperatures ranging from 50° to 150° C. Comparison tests were run in which acid-clay mixtures were cured at room temperature (25° C). Variables investigated were (1) amounts of acid added, (2) amount of water used to pelletize the clays, (3) temperature (either 25° C or baking temperature), and (4) method of mixing clay with acid and water. Results are tabulated in table 3, All acid additions are expressed as the pounds of equivalent 98-pct H2SO4 added per ton of clay feed material.
The table shows that at least 1,150 pounds of concentrated acid is required to solubilize over 80 pct of the lithium in 1 ton of McDermitt A. When this amount of acid was used, lithium extractions were 81 to 94 pct. These tests showed that the acid addition was the most significant variable; no other consistent trends were detected. Comparison of the acid baking results with those obtained by direct acid leaching shows that the two methods are equally effective for lithium extraction when an equivalent amount of acid is used.
Roast Tests
The McDermitt clay samples were treated by alkaline, sulfate, chloride, and multiple-reagent roasting followed by water leaching. To develop baseline data, samples of the clays were roasted without reagent additions at 1,000° C for 4 hours, and the roast calcines were leached with water. Analyses of the calcines showed that 24 pct of the lithium was volatilized from McDermitt A and 7 pct of the lithium was volatilized from McDermitt B during roasting. No lithium was extracted from either calcine by water leaching. It was assumed that the lithium volatilized from the clay during roasting could be either condensed as a solid product or collected as a soluble compound in an aqueous scrubbing solution. This assumption was confirmed in exploratory roasting tests conducted in an apparatus that was set up to collect all volatile products of the roasting reaction. Therefore, the percentages of lithium extracted that are reported for the following roast-leach tests are given as three values: lithium volatilized during roasting; lithium leached from the roast calcine; and total lithium extracted during the test.
Alkaline Roast Water Leach
The McDermitt clays were roasted with carbonates and hydroxides of sodium, potassium, and calcium, and the roasted calcines were leached with water. The purpose of these tests was to determine if the lithium in the montmorillonite clay structure can be displaced and converted to a water-soluble form by the alkaline roasting reactions.
One-hundred-gram samples of the clays were mixed with potassium, sodium, or calcium carbonate, and the mixtures were roasted at 1,000° C for 4 hours. The roasted calcines were ground and analyzed. Material balance calculations showed that 93 pct or more of the lithium in the feed material was present in the calcine; less than 7 pct of the lithium was vaporized during roasting. Samples of the roasted calcines were leached with water at 10 pct solids to extract soluble lithium. Results of carbonate roast-water leach tests are given in table 4.

The sodium carbonate roasts produced the highest lithium extractions: 44 pct from McDermitt A and 38 pct from McDermitt B, Roast tests with potassium and calcium carbonates produced lower lithium extractions.
The McDermitt clays were roasted with sodium hydroxide at 1,000° C. The roast products were a fused mass that attacked the roasting crucible. Over 72 pct of the lithium in the raw materials was volatilized as the oxide from the clay-hydroxide mixture during roasting. Only 1 pct of the lithium in the calcines was removed by water-leaching. Roasting tests were also run at 500° C. Both potassium and sodium hydroxides were used in these tests. Results, given in table 5, showed that no lithium was volatilized from the McDermitt A calcine and that only 5 to 8 pct was volatilized from the McDermitt B calcine. However, water-leaching these calcines extracted only 1 or 2 pct of the contained lithium.

Sulfate Roast Water Leach
McDermitt clays were roasted with calcium, sodium, or potassium sulfate to form water-soluble lithium sulfate. Mixtures of the clays and sulfates were roasted at 1,000° C for 4 hours, and the calcines were leached with water. Results of initial tests with sodium and potassium sulfates showed that less than 50 pct of the lithium was extracted from each sample. However, roasting tests with calcium sulfate produced lithium extractions of 62 to 68 pct. Therefore, further calcium sulfate tests were run to investigate the effects of temperature and amount of calcium sulfate used. Roast temperatures tested were 800° to 1,100° C. For these tests, 50 grams of calcium sulfate was mixed with 100 grams of clay. In one duplicate test at 1,000° C, one-half the standard quantity of calcium sulfate was used. When 100 grams of the clays were roasted with 25 grams of calcium sulfate instead of 50 grams, lithium extractions were reduced from over 60 pct from both clays to 15 pct from McDermitt A and to 50 pct from McDermitt B.
Results of the temperature tests, given in table 6, show that lithium extractions from McDermitt A and B are very dependent upon roasting temperature.
At optimum temperature of about 1,000° C, the total lithium extraction was 67 pct from McDermitt A and 72 pct from McDermitt B. Table 6 shows that the amount of lithium volatilized from McDermitt B was higher at 1,100° C than at 1,000° C, but the amount of lithium extracted from the calcine by water- leaching was much lower.

Chloride Roast Water Leach
A chloride roast-water leach process has been reported by Peterson and Gloss for extracting lithium from spodumene ores. In their process, 347 parts of potassium chloride was roasted with 100 parts of spodumene that contained 3.58 pct Li. The ratio of potassium chloride to lithium in the ore was 89 to 1. When chloride roast-water leach techniques were investigated for extracting lithium from McDermitt B, the same weight ratio was used. This weight ratio was 57 grams of potassium chloride to 100 grams of the clay. The same amount of potassium chloride was used in the roasting tests with McDermitt A; in these tests, the weight ratio of potassium chloride to lithium in the clay was 158 to 1.
The mixtures of clay and potassium chloride were roasted at 1,000° C for 4 hours. The calcines produced were leached with water to extract water-soluble lithium chloride. For McDermitt A, 27 pct of the lithium was volatilized during roasting but no lithium was extracted from the calcine by water-leaching; the total lithium extraction was 27 pct. For McDermitt B, 39 pct of the lithium was volatilized during roasting and, of the lithium remaining in the calcine, 31 pct was leached with water. The total lithium extraction was 58 pct-39 pct in the volatile product and 19 pct in the water-soluble product.
Multiple Reagent Roast Water Leach
The purpose of multiple-reagent roasts was to achieve greater lithium extraction by combining the effects of chloride roasting with those obtained by a roast-water leach treatment. Two combinations of reagents were tested: potassium chloride plus calcium carbonate and potassium chloride plus calcium sulfate. Variables investigated were the roasting temperatures and the amount of reagents added. These tests were conducted with McDermitt B only, because earlier tests had shown that lithium can be extracted from McDermitt by less complex procedures than the multiple-reagent roast-water leach treatment.
Potassium Chloride-Calcium Carbonate Roast-Water Leach
Conditions and results of potassium chloride-calcium carbonate roast tests are given in table 7.
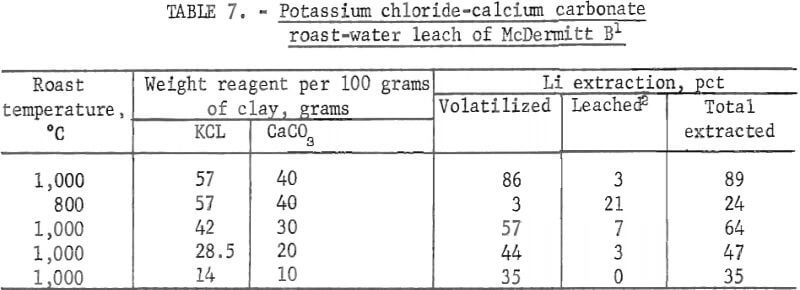
Potassium chloride-calcium carbonate roast-water leach treatment of McDermitt B was the most successful technique tested for extraction of lithium from this clay. A total of 89 pct of the lithium was extracted—86 pct as the volatile product and 3 pct as the water-soluble product, In contrast, the lithium extraction from McDermitt B by roasting with potassium chloride only was 58 pct, and the lithium extraction by roasting with calcium carbonate only was 18 pct. Results of the tests at 800° and 1,000° C indicate the mixtures must be roasted at the higher temperature in order to extract lithium as a volatile product. Tests with different amounts of reagents show that lithium extractions increased in proportion to the amounts of reagents added.
Potassium Chloride-Calcium Sulfate Roast-Water Leach
Conditions and results of potassium chloride-calcium sulfate roast-water leach tests are given in table 8.

Roasting McDermitt B with a combination of potassium chloride and calcium sulfate resulted in a total lithium extraction of 81 pct; roasting the clay with calcium sulfate only showed a total lithium extraction of 72 pct. The amount of lithium volatilized during potassium chloride-calcium sulfate roasting and during calcium sulfate roasting is the same (see table for calcium sulfate roast), but the amount of lithium extracted by water-leaching the calcines produced is 67 pct from the multiple-reagent roast as contrasted to 59 pct from the calcium sulfate roast. Again, roasting at 1,000°C is much more effective than roasting at 800° C, and lithium extractions are in proportion to the amounts of reagents added.
Roast Leach Treatment Summary
Exploratory extraction studies were conducted on two samples of lithium, bearing clays. The samples were from the McDermitt Caldera near the Oregon-Nevada border and represented an extensive deposit of hectorite raw material. One sample from the northern part of the deposit contained 0.36 pct Li and was designated McDermitt A; the second sample from the southern part of the deposit contained 0.64 pct Li and was designated McDermitt B, The extraction techniques tested were—
- Agitation leaching with sulfuric acid.
- Sulfuric acid bake-water leaching techniques.
- Roast-water leach procedures using carbonates, sulfates, chlorides, and combinations of these reagents.
Although the two clay samples were from the same caldera, they exhibited different processing characteristics. Lithium extraction from McDermitt A could be achieved by simple acid leaching, but lithium extraction from McDermitt B required more severe processing treatment.
Results of the extraction tests are as follows:
- Sulfuric acid leaching extracted over 90 pct of the lithium from McDermitt A but only 1 pct of the lithium from McDermitt B.
- Alkaline carbonate roast-water leach treatment extracted about 40 pct of the lithium from each clay.
- Both alkaline hydroxide roast-water leach treatment and calcium sulfate roast-water leach treatment extracted about 70 pct of the lithium from each clay.
- Potassium chloride roast-water leach treatment extracted 27 pct of the lithium from McDermitt A and 58 pct of the lithium from McDermitt B.
- Lithium extractions from McDermitt B were significantly increased when multiple-reagent combinations were used. Potassium chloride-calcium carbonate roast-water leach treatment extracted 89 pct of the lithium, and potassium chloride-calcium sulfate roast-water leach treatment extracted 81 pct of the lithium.
Recommendations about Roast Leach Treatment
The extraction studies illustrate that results from tests with one clay cannot be used to predict the results that will be obtained when the same extraction technique is applied to different clay. The two samples from the McDermitt Caldera responded differently to the extraction techniques tested. However, the exploratory test results can give general directions for further work.
Techniques that appear to warrant development for extracting lithium from the McDermitt clays are (1) sulfuric acid bake-water leach; (2) chloride roast-water leach; (3) sulfate roast-water leach; and (4) multiple-reagent roast-water leach. The developmental investigations must be designed to yield results that can be used to evaluate both the economic feasibility and the environmental impact of the process.
Further work on the sulfuric acid bake-water leach extraction technique should include:
To evaluate economic feasilility:
- Recycling tests of water leach solutions to obtain high lithium concentrations in solution while still achieving acceptable extraction from the clay.
- Countercurrent leaching-washing operations to remove entrained leach liquors from the solid residues.
To evaluate environmental impact:
- Analyses of the final solutions and solid residues produced to determine levels of accessory (trace) elements.
- Purification studies for effective removal of prohibited accessory elements from solutions if analyses show them to be present at higher than acceptable levels.
- Percolation tests on the insoluble residue from water leach operations to determine the nature and amount of salts that will be leached into ground waters by rainfall and melting snow.
Further work in the reagent roast-water leach extraction technique should include:
To evaluate economic feasibility:
- Roasting tests to determine the optimum conditions of time and temperature and to determine the minimum amounts of reagent additions required for lithium extraction.
- Roasting tests to determine the effect of different furnace atmospheres on recovery of volatile products.
- Leaching tests to investigate efficient methods for obtaining higher lithium concentrations in leach liquors.
To evaluate environmental impact:
- Analysis of the offgases produced during roasting to determine whether the gases must be scrubbed to remove pollutants.
- Percolation tests on the water leach residue to determine whether salts will be leached into ground waters when the residue is returned to the surrounding area.
The Federal Bureau of Mines investigated the extraction of lithium from hectorite-type clay deposits found on the Nevada-Oregon border. Two clay samples were used in the investigation; one contained 0.36 pct Li and the second contained 0.64 pct Li. The purpose of this laboratory-scale research was to determine how these clays responded to different extraction techniques. Extraction techniques investigated were sulfuric acid leach, sulfuric acid bake-water leach, and roast-water leach procedures with cholorides, sulfates, carbonates, and combinations of these reagents. The processing characteristics of the clay samples showed significant variations, and no one technique was effective for both materials. Lithium extractions near 90 pct were achieved during these exploratory studies.
