Table of Contents
The extraction of gold from ores that contain carbon and organic compounds has presented a problem to the cyanide mill operator ever since the widespread adoption of this classical dissolution technique in the late 1800’s. The problem was studied in 1924 by Leaver and Woolf. More recently, Hedley and Tabachnick indicated that a general solution to the problem was not available.
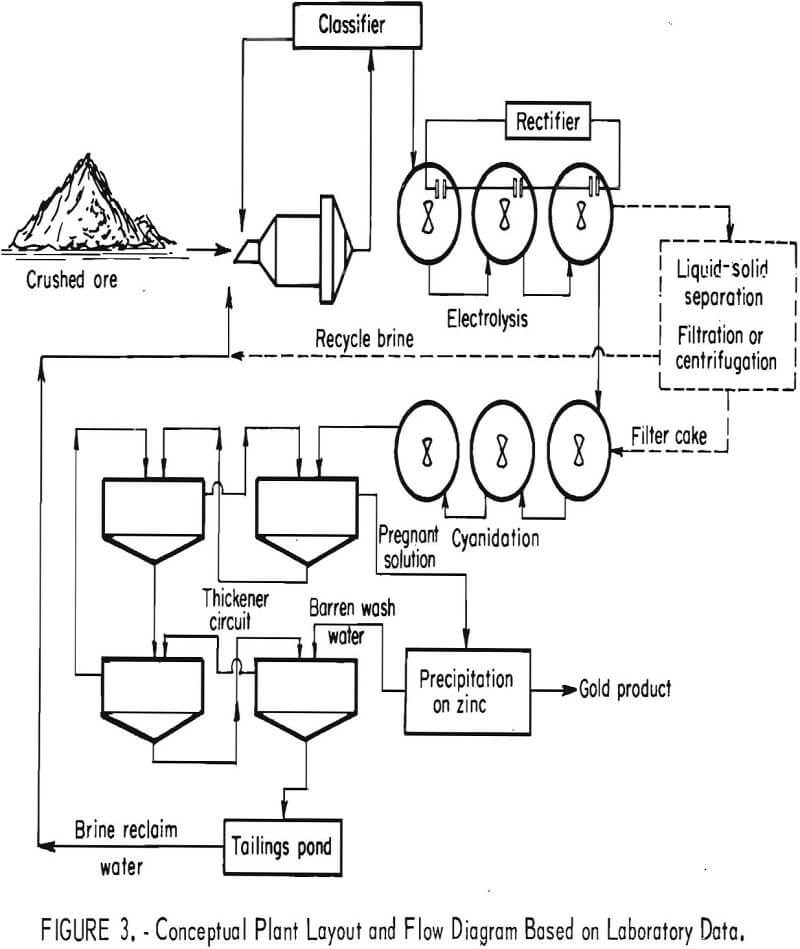
Treatment of these gold ores was investigated on a laboratory scale by the Bureau of Mines. Carbon components in the ore were shown to interfere with gold recovery by adsorbing the Au(CN)2- complexes, causing gold to be lost in process tails. Oxidation treatment by a hypochlorite sequence was shown to be effective for destroying the adsorptive properties of the carbonaceous ore for the gold cyanide complex. Methods of applying hypochlorite oxidation to the ores included (1) addition of sodium hypochlorite, (2) chlorine addition, and (3) generation of hypochlorite in situ by electrolysis of ore pulp containing sodium chloride. A conceptual flow diagram for treatment of carbonaceous gold ores by oxidation was developed as a result of the investigations.
This report is concerned with pilot, plant studies on the process conducted on a cooperative basis between the U.S. Bureau of Mines and the Carlin Gold Mining Co., a wholly-owned subsidiary of the Newmont Mining Corp. Studies were conducted initially at the Reno Metallurgy Research Center, and finally at the Carlin gold mine. The objective was to determine technical and economic feasibility of applying the laboratory-developed process on a commercial basis to carbonaceous gold ores occurring with oxide ores in the Carlin open pit gold mine. Pilot plant studies included oxidation procedures using sodium hypochlorite, chlorine, and electrooxidation.
Materials Equipment and Procedures
Principal studies were conducted on ores from the Carlin gold mine. These ores included a typical oxidized gold ore, to standardize the cyanide portion of the pilot mill, and three carbonaceous gold ores. The gold and organic carbon contents of the four ores are given in table 1.
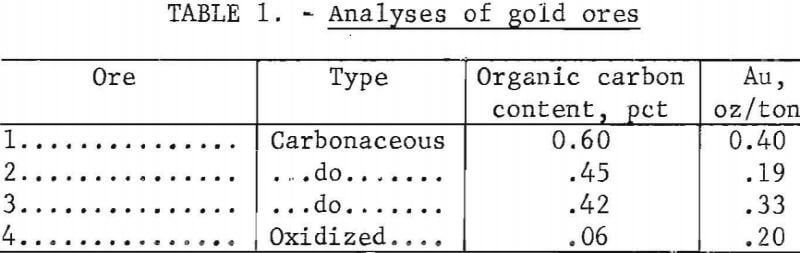
The ores have been described in detail elsewhere. They consisted essentially of hydrothermally altered, silty, dolomitic limestone, partially replaced with microcrystalline quartz. The carbonaceous materials consisted of graphitic-type carbon, activated-type carbon, and organic compounds similar to humic acids that appear to be capable of complexing gold ion. The gold present, in these ores was in the microscopic to submicroscopic size range (0.2 micron or less), ‘The general appearance of the oxidized and carbonaceous ore is shown in figure 1.
Pilot plant experiments were conducted in the apparatus shown in figure 2. Equipment (some not shown) consisted of an 18-in by 3-ft rod mill, three rubber-lined 55-gallon oxidation tanks fitted with electric heating coils and marine-type agitators, a 55-gallon mild steel digestion-surge tank, three mild steel 55-gallon cyanidation tanks fitted with marine-type agitators, four 3-foot by 3-foot thickeners, and a precipitation unit.

NaOCl was added to the ore pulp by metering the desired amount of a 5-pct NaOCl solution into the oxidation tank during the selected time interval.
For experiments utilizing chlorine as the oxidant, pulp was passed through the oxidation system semicontinuously and chlorine gas was bubbled into the ore pulp through a section of plastic pipe extending to the bottom of each oxidant tank. The rate of chlorine gas addition was controlled by monitoring the loss in weight of the chlorine storage bottle.
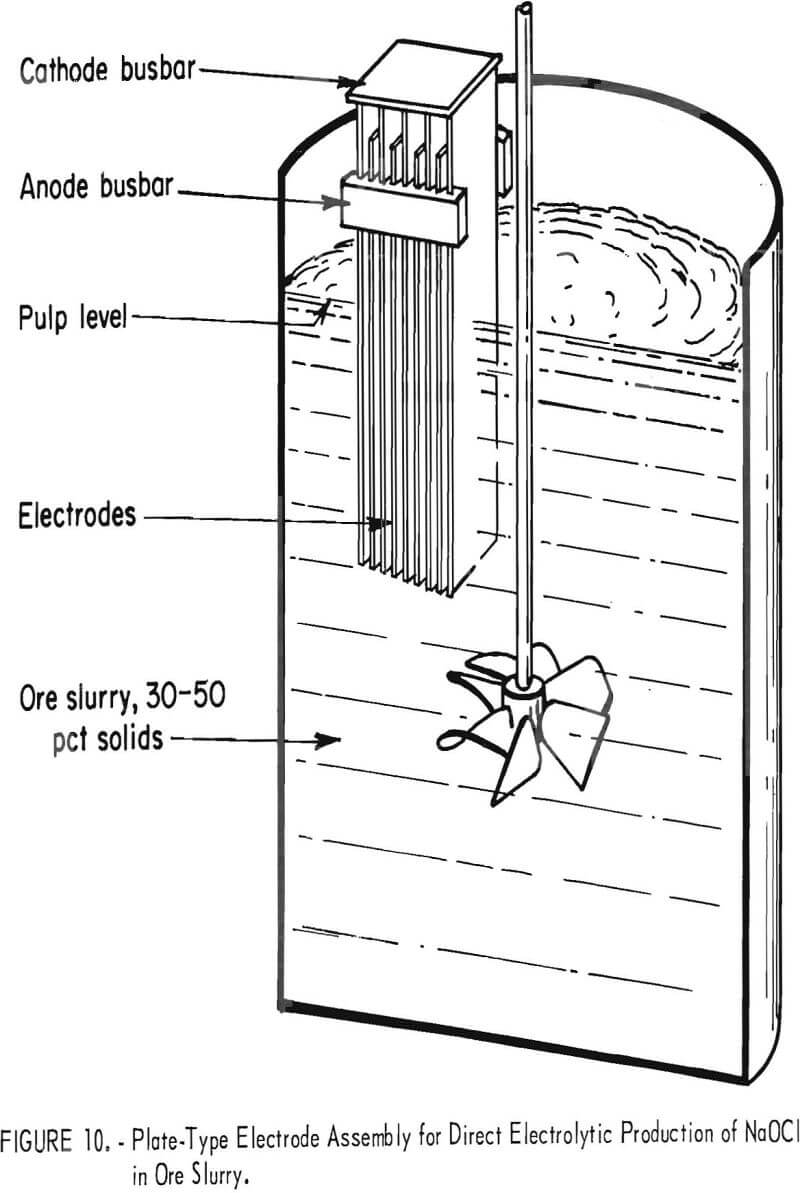
For experiments generating hypochlorite in situ by electrolysis of ore pulped with brine, pulp was passed through the oxidation system continuously. Each of the three oxidation tanks was fitted with a series of electrodes consisting of three graphite anodes and four graphite cathodes with an electrode spacing of ½ in. Each electrode was 2-½ in wide, ¾ in thick, and 30 in long. Electrodes were immersed a nominal 20 in below the surface of the pulp. Direct current was supplied by a rectifier.

Equipment was generally arranged according to the conceptual flow diagram developed in laboratory studies (fig. 3), with minor modifications required to accomplish oxidation by the procedures investigated.
Flow sequence and operation in the pilot plant generally followed conventional countercurrent decantation slime circuit operating practice. Material was ground to 60 pct minus 200 mesh at 50 pct solids in the rod mill and pulped into the oxidation tanks, each of which held 2.75 lb of dry ore (550 lb of pulp). After treatment in the oxidation tank at the desired temperature, the pulp was passed through the digestion-surge tank where 1 lb of cyanide per ton of ore was added, along with sufficient lime to maintain a cyanidation pH of 11. Approximately 5 lb of lime per ton of ore was usually required to maintain the desired pH value. The pulp was then pumped through the three cyanidation tanks, for a cyanidation time of 9 hr. From the cyanidation tanks the pulp passed through the four thickeners at 20 pct solids, flowing countercurrent to the barren solution recycled from the gold precipitation sequence. Pregnant solution was passed from the first thickener to the gold precipitation system.
Gold recovery from solution was accomplished principally by conventional Merrill-Crowe precipitation on zinc. Adsorption of the Au(CN)2- on Rohm and Haas IRA 425 ion exchange resin was used for convenience during selected experiments. The adsorbed gold was stripped from the resin with an acetone solution, containing 5 pct HCl and 5 pct water, and then precipitated with zinc.
Heads and tails analyses were obtained by the conventional fire assay method, and pregnant solution analyses were obtained by an atomic absorption method.
Results and Discussion
Initial pilot plant experiments were conducted on nonrefractory oxide gold ore, described previously, to determine major operating conditions of grinding circuit operation, pulp handling, flow rates, etc. Mill tails from the oxide ore contained 0.008 oz gold per ton, which corresponds to a gold extraction of 96 pct.
Carbonaceous gold ore 1 was processed in the pilot mill, using conventional cyanidation, without oxidation pretreatment, as a base line experiment to determine the effect of carbonaceous material. Gold extraction obtained in these experiments ranged from 29 to 33 percent (0.26 to 0.28 oz gold/ton tails).
Oxidation by NaOCl Addition
Pilot plant operations were conducted on 21 tons of carbonaceous ores 1 and 2, using sodium hypochlorite as the oxidant to destroy the adsorbing properties of the carbonaceous matter.
Operating conditions established in laboratory experiments for obtaining favorable gold extraction values were followed generally in the pilot plant operations. The oxidation section of the plant was operated on a semicontinuous basis with ground ore being treated on a batch basis in successive oxidation tanks. The ore could then be discharged into the surge tank in such a manner that a reserve of treated pulp was available for continuous operation of the cyanidation and liquid-solids separation sections of the mill. This section of the mill processed 80 lb of dry ore per hr, thus providing a retention time of 9 hr in the cyanidation tanks.
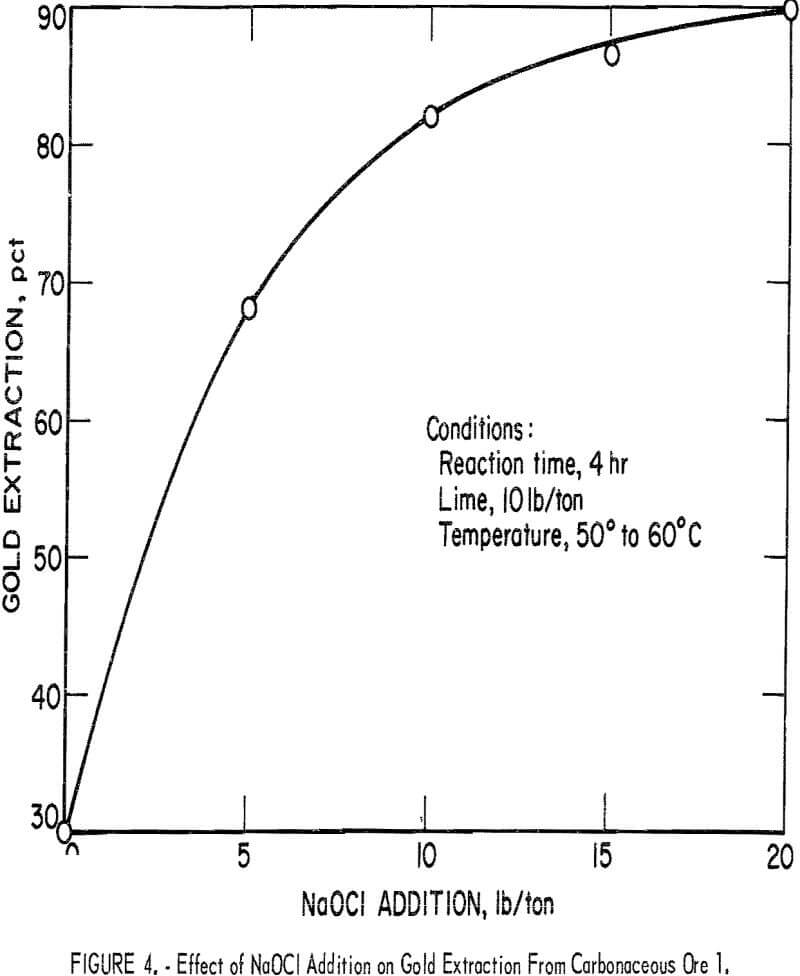
Figure 4 shows the oxidation and subsequent gold extractions obtained at different levels of NaOCl addition to the pulp containing carbonaceous ore 1. Constant conditions of a 4-hr reaction time, 10 lb lime per ton ore, and a temperature of 50° to 60° C were maintained. Corresponding pH values were in the 11 to 11.5 range. Extraction increased markedly on addition of 5 to 10 lb of NaOCl and increased gradually with larger NaOCl increments. Gold extraction obtained by subsequent cyanidation reached 90 pct with addition of 20 lb NaOCl per ton ore. Results closely paralleled similar laboratory experiments conducted on a different sample of carbonaceous gold ore.
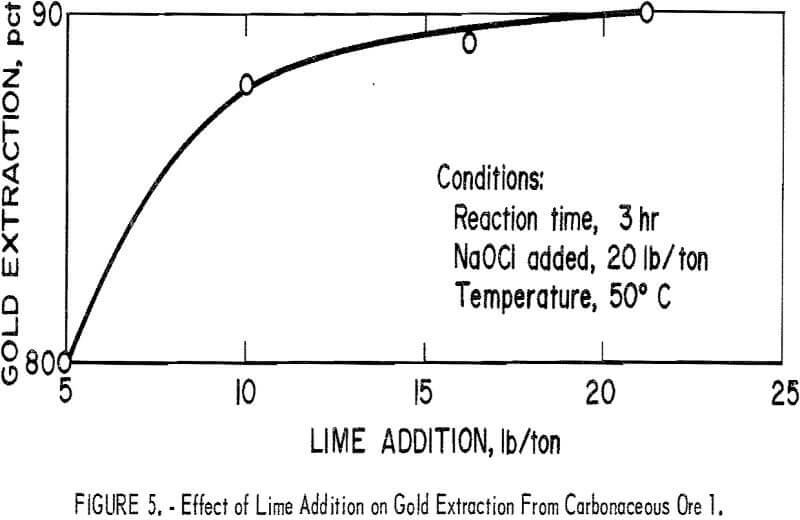
To determine the effect of lime addition on oxidation and the consequent gold extraction obtained by cyanidation of the treated pulp, a series of experiments were conducted by adding different quantities of lime to the pulp. The desired amount of lime was added to each slurry and stirred for 1 hr before addition of NaOCl for oxidation. Constant conditions of 20 lb NaOCl per ton ore, 3-hr reaction time, and 50° C were maintained using carbonaceous ore 1. The initial pH of the pulp was essentially constant at pH 11 to 11.5 on addition of the 5 to 20 lb of lime per ton ore, and then decreased to approximately pH 9 for 5 and 10 lb of lime and pH 10 for 15 and 20 lb of lime per ton. Figure 5 shows that the amount of lime used in the system had a moderately significant effect on gold extraction by subsequent cyanidation; extraction increased from 80 pct with 5 lb lime per ton ore to 90 pct with 20 lb lime per ton ore.
The temperature at which oxidation was conducted was determined to be an important variable in laboratory experiments, since it affected both the rate of oxidation reaction and decomposition of the sodium hypochlorite. Pilot plant experiments to determine the magnitude of these effects were conducted at constant operating conditions of 20 lb NaOCl per ton, 10 lb lime per ton,
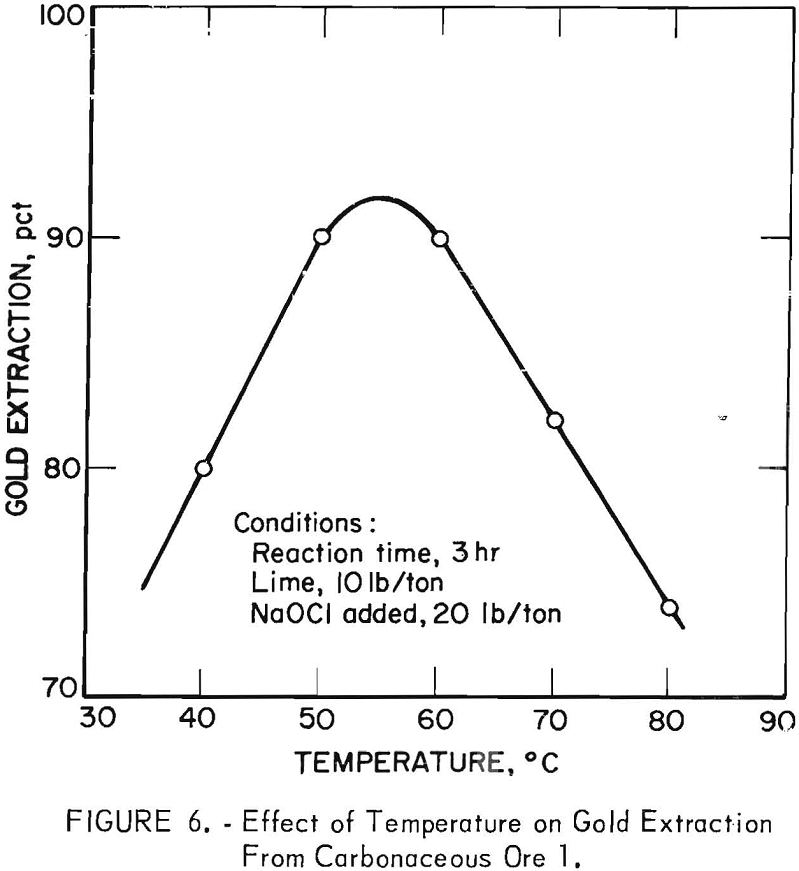
a 3-hr reaction time. Initial pH was 11.5 and final pH equaled 10. Figure 6 shows that maximum gold extraction was obtained by cyanidation of pulps oxidized at temperatures between 50° and 60° C.
Laboratory experiments had indicated that the rate of addition of hypochlorite to the system could be an important variable. High concentrations of hypochlorite in the presence of the pulp could result in hypochlorite being wasted in oxidizing ore components not deleterious to cyanidation, and decomposition of unreacted hypochlorite could occur at temperatures in the 60° C range. Pilot plant experiments conducted on this facet of the investigation were carried out with 10 lb lime per ton ore, and 3-hr-digestion time at 60° C. Twenty pounds of sodium hypochlorite were added to the pulp in increments, and the rate of addition was varied during the treatment period. The pH of the pulps was in the 11 to 11.5 range initially and then decreased to 9 to 9.5 as the oxidation proceeded. Results indicated that the technique enhanced extraction by decreasing the gold remaining in the tails to 0.015 oz gold per ton tails, but that the time interval between NaOCl additions was not critical. Initial addition of 10 lb NaOCl per ton ore to the pulp and a 2-hr reaction time appeared to be sufficient to give favorable extraction.
Oxidation by Chlorine Addition
Addition of chlorine to a pulp of finely ground carbonaceous ore and water was investigated as a means of producing hypochlorite oxidant in situ on an economical and easily controlled basis. As chlorine is bubbled into the pulp, it reacts with water to form hypochlorous acid and hydrochloric acid. These products are buffered by the calcareous gangue in the ore to form calcium hypochlorite and calcium chloride. Oxidation of the carbonaceous matter is thought to be accomplished by the hypochlorite ion.
Pilot plant experiments generally were conducted under conditions that closely paralleled those determined to be near-optimum for gold extraction in bench-scale laboratory experiments. It had been shown in laboratory experiments that addition of chlorine gas at moderate rates to the agitated pulp through a sintered-disk sparger resulted in favorable oxidation without excessive loss of chlorine gas. The chlorine reacted rapidly and gold extraction from the oxidized pulp by subsequent cyanidation was shown to be largely independent of the rate of chlorine addition. The factor limiting the rate of chlorine addition appeared to be the amount of hypochlorite remaining after oxidation. The hypochlorite product reacted with the carbonaceous matter as chlorine was added during the initial stages of the oxidation and no hypochlorite could be detected in the solution. The amount of hypochlorite in solution increases with time until the oxidation is complete. It has been determined that the oxidation can be completed in as little as 4 hr; however, at this relatively rapid rate of chlorination, the Ca(OCl)2 in solution can build up to 1 pct or more in the later stages of oxidation. If hypochlorite is allowed to build up to a level which cannot be consumed by the ore, it will consume cyanide in the cyanide circuit. Therefore, it was desirable to limit the calcium hypochlorite in solution to less than 0.2 pct during chlorination. This resulted in a chlorination time of up to 15 hr at 24° to 30° C for some of the more refractory samples, with an additional 5 hr for the ore to consume the residual hypochlorite. The final concentration of calcium hypochlorite going to cyanidation was less than 0.02 pct.
Heating of the pulp to maintain a minimum temperature of 24° C was necessary to obtain a reasonable rate of oxidation of the carbon compounds while maintaining the hypochlorite at less than 0.2 pct. For example, in one experiment, a total of 33 hr was required for oxidation at 18° to 22° C, whereas only 14 hr were required for the same amount of oxidation at 24° to 27° C.
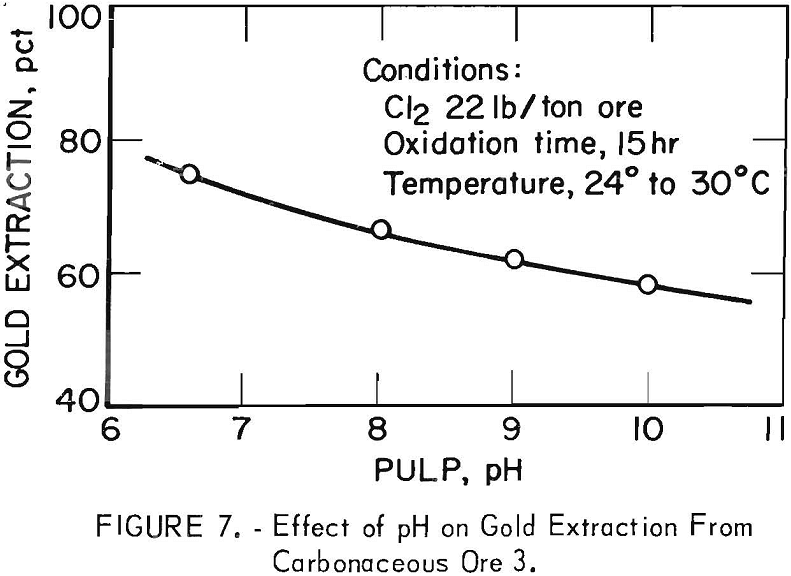
The pH of the oxidizing solution possessed a moderate but definite effect on the degree of gold extraction obtained by subsequent cyanidation. Figure 7 shows that when a fixed oxidation time of 15 hr was used, the gold extraction decreased almost linearly with increasing pH from the starting pH of 6.6 obtained without addition of lime. This effect of pH is opposite to that observed previously in figure 5 with addition of NaOCl. The reason for the divergent effect of pH in the two systems is not clear. The acid generated during chlorine addition may serve to liberate the gold from the calcareous gangue more readily. Conversely, at higher pH values, some of the hypochlorite may be converted to chlorate, which is not an oxidizing agent in basic media.
Pilot plant tests were run with three 55-gallon drums connected in series, each containing 275 lb solids at 40 pct solids. Chlorine was fed to each drum through a ½-in pipe extending to the bottom of the drum. Oxidation was conducted on a batch basis with the pulp overflow from the third tank being recirculated back through the system continuously to give the desired chlorination time.
Seven tons of carbonaceous ore 3 were treated in the pilot plant to determine the effects of amount and rate of chlorine addition and temperature on gold extraction. The pH of the system remained at 6.6 during chlorination without adjustment. The importance of temperature and rate of chlorine addition as determined in laboratory experiments was confirmed in pilot plant experiments. A temperature of 24° to 30° C in the chlorination pulps was considered to be near-optimum for gold extraction by subsequent cyanidation. A chlorine addition rate corresponding to 15 hr treatment time with sufficient chlorine for favorable extraction gave the desired residual hypochlorite value of less than 0.2 pct. During the subsequent 5 hr of digestion, the hypochlorite concentration dropped to less than 0.02 pct.
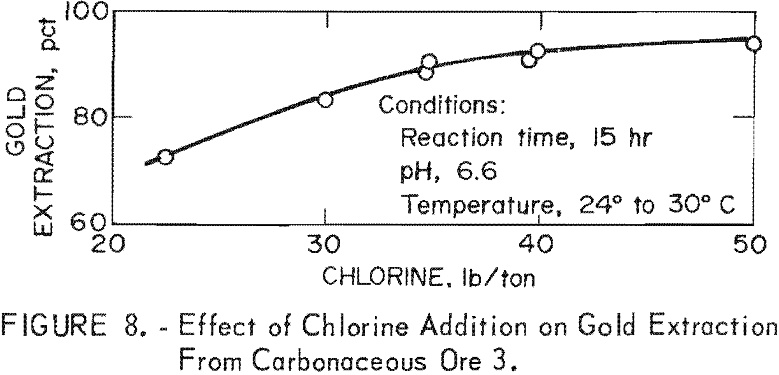
The effect of the amount of chlorine on gold extraction was determined in a series of experiments conducted at constant conditions of 24° to 30° C, 15 hr chlorination time, and pH of 6.6 on carbonaceous ore 3. Figure 8 shows that gold extraction increases rapidly with increasing chlorine addition up to 92-pct extraction at 40 lb chlorine per ton ore and then levels off on further chlorine addition. The results closely paralleled laboratory data with this particular ore.
Pilot plant studies were continued with the specific objective of determining if impurities built up in the recycled barren solutions would cause difficulties with gold precipitation. Carbonaceous ore 3 was oxidized with chlorine under constant conditions of 24° to 30° C, 15 hr chlorination time, pH of 6.6 and 30 lb chlorine per ton ore. The oxidized carbonaceous pulp was mixed with mill pulp (oxide ore) in a ratio of 1 carbonaceous to 6 mill pulps. The mixed pulps were cyanided for 16 hr and the pregnant solution was removed by decanting and washing. The pregnant solution was then precipitated by the standard zinc method. A second portion of carbonaceous ore was oxidized under identical conditions, mixed with mill pulp (1 to 6) and cyanided. The pulp was mixed in a thickener with the wash solution from the first oxidation. This pregnant solution was decanted, and the ore was washed with the barren solution from the first oxidation. The sequence was repeated nine times using the same barren and wash solutions each time. Precipitation results were excellent for all nine pregnant solutions (<0.001 oz gold per ton barren solution). No buildup of deleterious impurities was observed.
Scale-up experiments were conducted in a 6- by 7-ft tank containing 7.5 tons of pulp at 40 pct solids. Chlorine gas was bubbled in through four pipes submerged 5 ft into the tank. Chlorine was added over an 8-hr period at the rate of 3,5 lb per ton per hr initially, and decreased to 1.5 lb per ton per hr over the last 2.5 hr. Gold extractions of 89 pct (0.035 oz gold per ton tails) were obtained using 38 lb chlorine per ton. These results paralleled those obtained on the pilot plant scale experiments.
Electrooxidation
Electrolysis was investigated as a means of generating oxidizing conditions in situ in the ore pulp prepared from finely ground carbonaceous ore and NaCl brine. This would result in (1) potential reduction in costs incident to purchase of hypochlorite and chlorine reagents, as compared to the costs of the power and salt; and (2) producing the required oxidant at the desired rate so that excess oxidant would not be consumed by the ore.
Since hypochlorite oxidant is the product of electrolysis in the pulp, the factors that affect the efficiency of its production are critical in the electrolytic procedure. Design of the electrode system, salt concentration, conductivity of the ore-brine pulp, rate of electrolysis, time, temperature, and current density were all considered important variables.
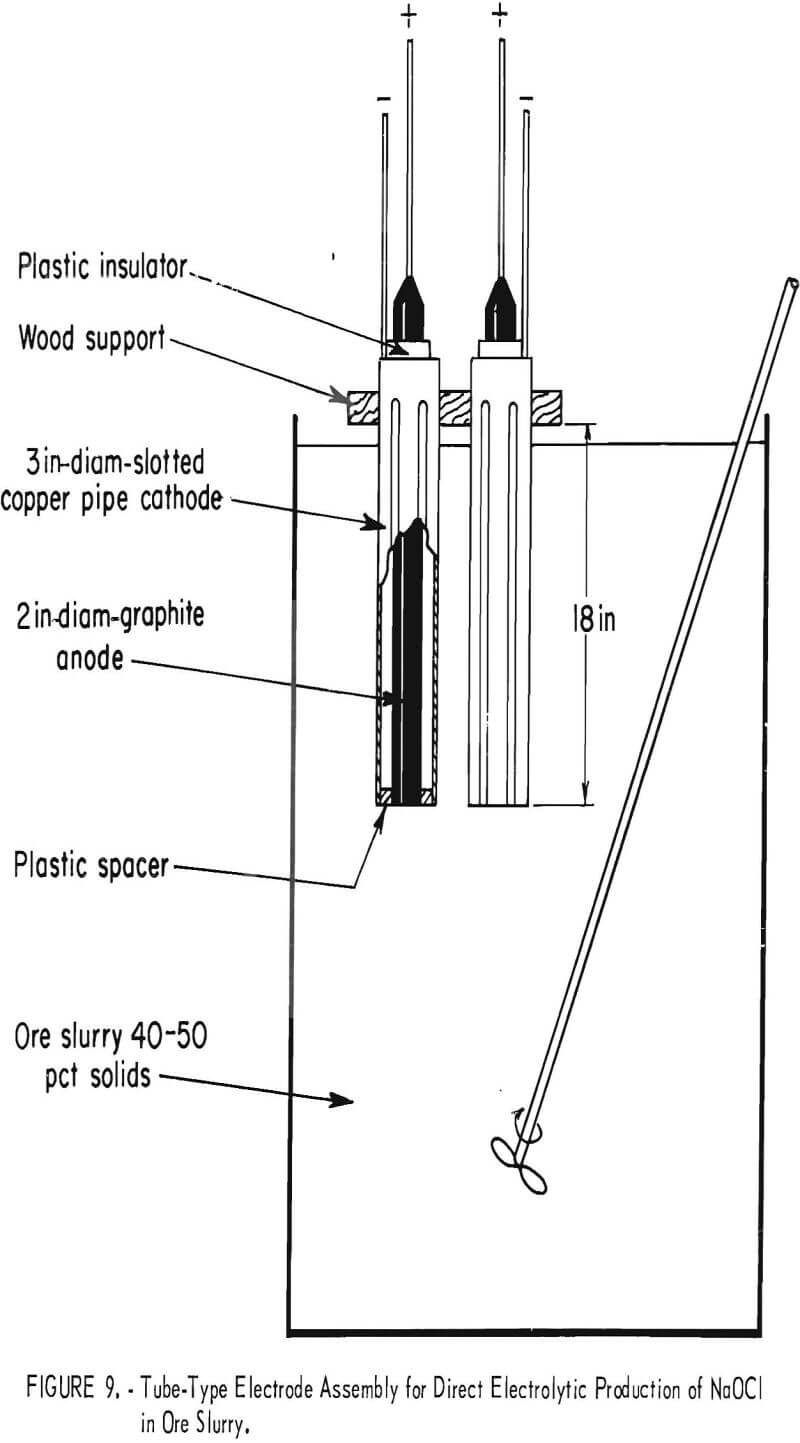
Three basic electrode systems were investigated in initial experiments. The first electrode design, shown in figure 9, was based on the use of tubular electrodes, consisting of 18-in lengths of 3-in-diameter copper pipe cathodes with 2-in graphite anodes positioned in the center of the pipe. Slots were cut lengthwise in the cathode to allow pulp to circulate between the electrodes. Preliminary experiments with this electrode system indicated that considerable resistance to pulp flow was encountered, resulting in a buildup of pulp inside the cathode pipe.
The second electrode system (fig. 10) was a simple plate-type arrangement consisting of graphite electrodes 2-½ in wide, ¾ in thick, and 30 in long. Identical graphite cathodes and anodes were placed alternately in a nonconductive holder with the ½-in spacing that allowed favorable pulp flow through the system. This electrode system was used basically for all pilot plant runs.
Graphite electrodes require an 8- to 10-pct salt concentration in the pulp solution to prevent degradation of the anode; therefore, a third electrode system utilizing PbO2- coated titanium electrodes as anodes and iron as the cathode was investigated. The PbO2 anode can be used for electrolysis in a 4-pct salt solution without degradation of the anode; however, higher power consumptions were encountered with the lower salt concentration.
During initial pilot experiments at the Carlin gold mine, it was observed that barren solutions, obtained from precipitating the gold on zinc, were inordinately high in gold content. Whereas the normal plant barren solutions may contain 0.001 oz gold per ton solution, barren solutions obtained by
precipitating the pregnant solution from cyaniding the oxidized carbonaceous ore contained as much as 0.015 oz gold per ton solution. Extensive analysis of pilot mill solutions and pulps showed the presence of chromium in pregnant solution in the amount of 3 to 8 ppm. Nickel was also present in smaller quantities. Bench-scale precipitation tests showed that chromium present in
pregnant solutions in excess of 1 to 1.5 ppm could adversely affect precipitation of the gold. It was known that the ore had less than the required amount of chromium to result in 8 ppm in the pregnant solutions; consequently, a detailed examination of the equipment was initiated to determine the source of chromium. The rubber coating on one of the stainless steel marine-type stirrers in an electrooxidation cell was found to be damaged, allowing the chromium in the alloy to be anodically dissolved. The chromium hindered gold precipitation. The reaction mechanism involved, however, was not investigated.
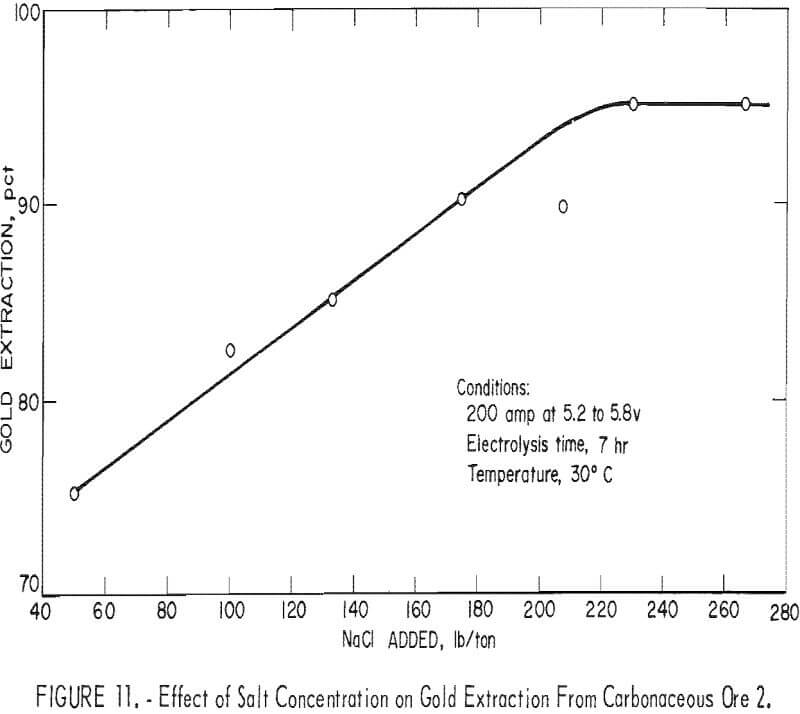
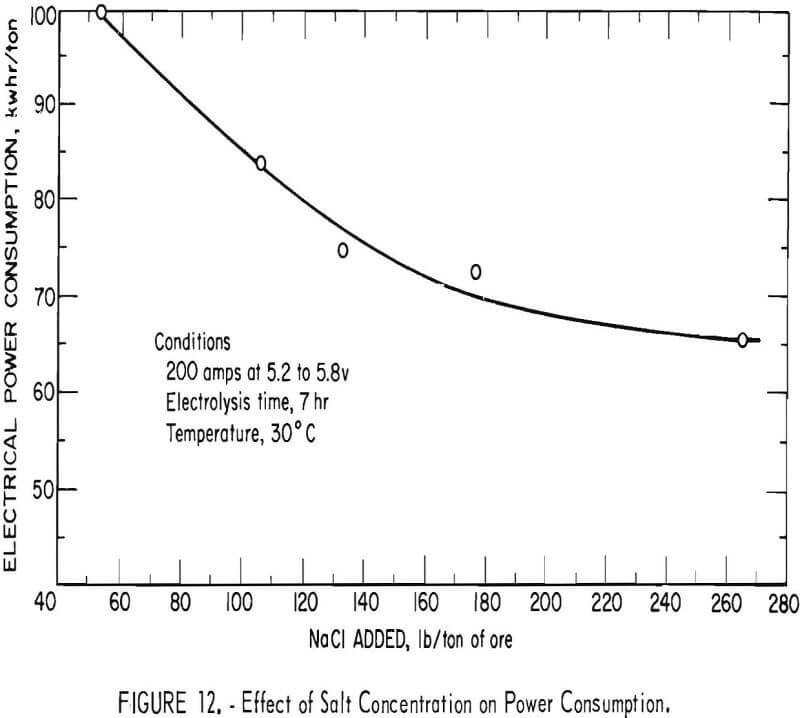
The concentration of salt maintained in the pulp solution is a principal variable since it affects the conductivity of the pulp and, consequently, the efficiency of hypochlorite production. Laboratory experiments had indicated that the use of an to 10-pct salt concentration in the pulp solution was desirable for favorable extraction. Pilot plant investigations of this variable were conducted over the range of a 1.7- to 8.9-pct salt concentration in the pulp solution. Constant operating conditions of 200 amp, current density of 0.55 amp per in², at 5.2 to 5.8 v and 40-pct pulp density were maintained with an electrolysis time of 7 hr using carbonaceous ore 2, Figure 11 shows that gold extraction after cyanidation of the oxidized ore increased almost linearly with increasing salt concentration. The use of salt concentration in excess of 200 lb per ton of ore resulted in gold extraction in the 95-pct range (0.01 oz gold per ton tails). Figure 12 shows that corresponding power requirements decreased from 100 kwhr per ton of ore at a 1.7-pct salt concentration to 65 kwhr per ton of ore at 8.9 pct salt, at which point gold extraction in the 95-pct range (0.01 oz gold per ton tails) was achieved.
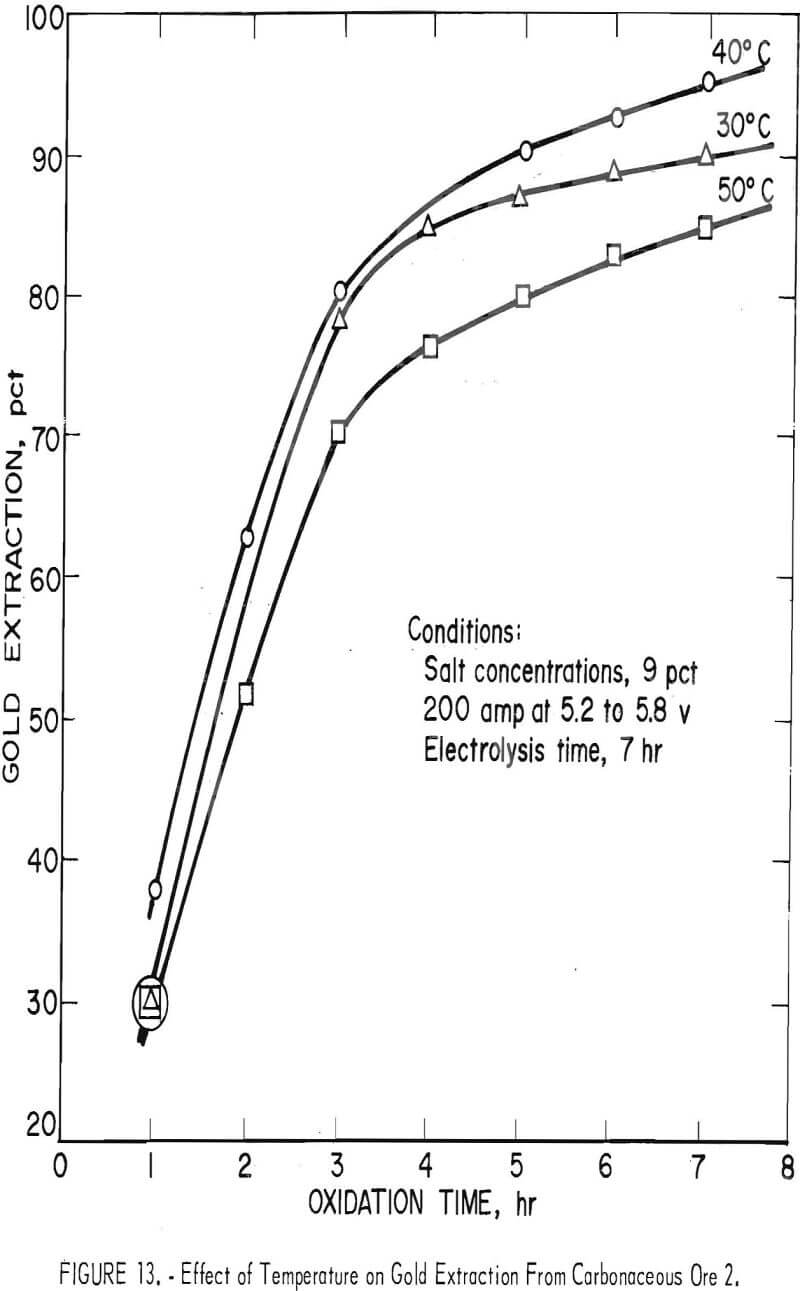
The effect of electrolysis time at different temperatures on gold extraction was determined in a series of experiments conducted at a constant current and 9-pct salt concentration. The data given in figure 13 indicate that an operating temperature of 40° C is near optimum for the production of hypochlorite and for oxidation of the carbonaceous matter in the ore. The maximum in gold extraction at 40° C is attributed to the increased decomposition rate of hypochlorite at temperatures above 40° C and a decrease in rate of oxidation of carbonaceous matter below 40° C. Since the amount of hypochlorite produced by electrolysis is a direct function of time, the increase in gold extraction with respect to time of electrolysis shown in figure 13 is expected. The reason for the apparent change in oxidation rate shown by the break in the curves was not investigated.
As current density increases, the voltage required for a fixed current increases. The result of high current density is excessive power consumption and increase of heat input into the system. High current densities also cause undesirable electrode reactions that produce NaClO3 in the electrolytic cell. Since the beneficial effects obtained by lowering the current density are limited by the number of cells that can be placed in an agitation tank, a compromise must be made for a practical operating procedure. The data indicated that a current density of 0.5 to 0.75 amp per in² of anode surface was acceptable.
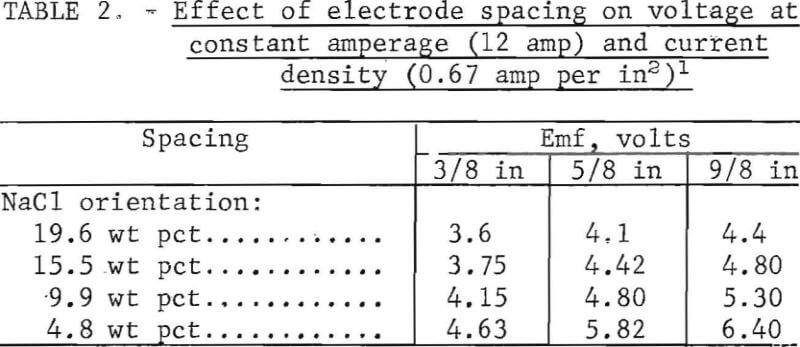
The salt serves as a good conductor; however, the solid particles in the pulp inhibit current flow so that pulp density becomes an operating variable in the production of hypochlorite oxidant. The data in figure 14 are the average of two experiments to determine the effect of pulp density on conductance. The measurements were taken with a standard conductance bridge. The data show a dramatic change in conductance with change in pulp density. A decrease from 0.175 mho conductance in a 10-pct salt solution (0 pct pulp density) to 0.066 mho for a 10-pct salt solution at 60 pct solids was obtained. From this, it appears that the lower pulp densities are better; however, in plant-size equipment, 40 pct solids is approximately the minimum that can be agitated satisfactorily for plant operation.
Data were obtained on the spacing between anode and cathode, and its effect upon the voltage-amperage relationships. Table 2 shows the voltage-amperage relationship at three different spacings for various salt concentrations maintaining a constant temperature of 35° C, 40-pct pulp slurry, and an 18-in² electrode area for both cathode and anode. The figures show that as salt concentration decreases, the spacing between the cathode and anode becomes increasingly important. Using 12 amp or a current density of 0.67 amp per in² as a standard, the relationship between spacing, salt concentration, and voltage is readily apparent (table 2). The difference in voltage between a 3/8-in and a 5/8-in spacing is only 0.5 volt at 19.6 pct salt, but 1.19 volts at 4.8 pct salt. An electrode spacing of 0.5 in would be satisfactory in operating practice.
At this point in the investigation, it became necessary to study two alternatives connected with simulating the operation of a commercial carbonaceous gold ore treatment plant In conjunction with the existing 2,400-ton-per-day oxide ore cyanidation mill. One alternative would be for the treatment plant to employ pulp filtration so that the brine solution would be totally recycled in the oxidation plant, with the washed filter cake being sent to cyanidation with the oxide ore. This would maintain the cyanidation section of the mill essentially salt free. The other alternative would be to pass the oxidized pulp containing NaCl directly to cyanidation with the oxidized ore, thus allowing solutions throughout the entire mill to contain 10 pct salt.
Experiments based on the first alternative were conducted on carbonaceous ore 3. The oxidized pulp was filtered and the brine electrolyte solution was recycled through the system to determine if a buildup of components deleterious to electrolysis would occur and the amount of salt lost to the filter cake. The filter cake was then cyanided with oxide ore in the ratio of 1 part of oxidized carbonaceous ore
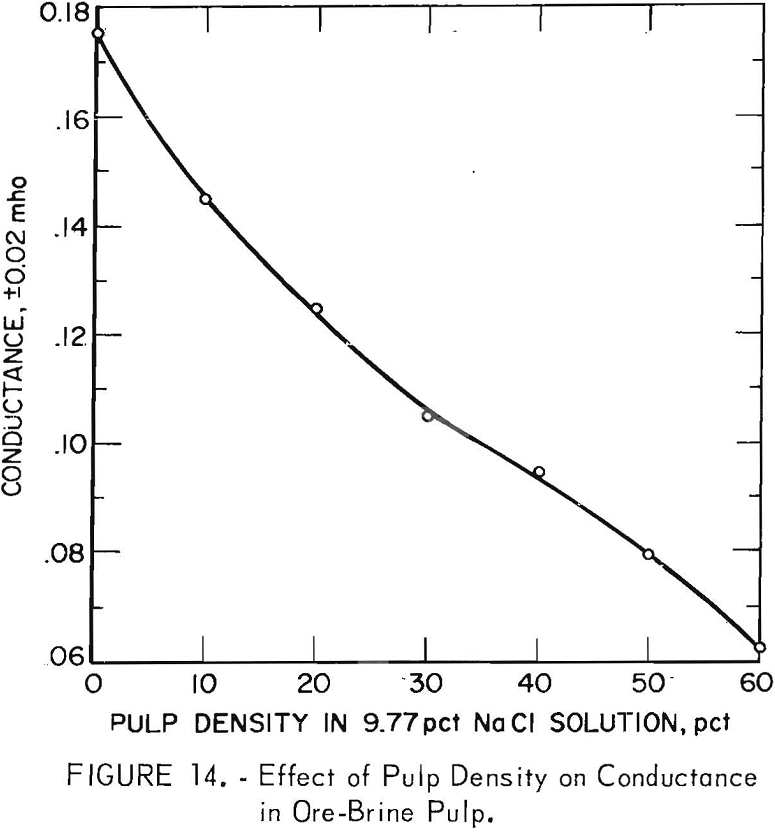
to 6 parts of oxidized ore. Mill solution was used in the cyanidation, and the barren solution obtained from precipitation of the resulting pregnant solution was used for wash water in the countercurrent decantation (CCD) circuit. Figure 15 is a simplified block flow diagram for the system.
No buildup of impurities was noted with 10 recycles of the brine solution in the electrolysis circuit and the barren solutions in the CCD circuit. Precipitation of gold from the pregnant solutions in the conventional Merrill-Crowe sequence was favorable in that barren solutions contained less than 0.001 oz gold per ton solution.
The drum filter used in the recycle experiment was a 9-ft² rotary drum filter. Filtering rate obtained during experiments with 45-pct pulp density was 26 lb per ft² per hr. Salt losses to the filter cake were 40 to 45 lb per ton ore. However, the cake was not washed with fresh water. A maximum filtering rate of 52 lb per ft² per hr was obtained with a 52-pct solids feed containing 0.20 lb of Betz 1320 flocculating agent per ton of ore at pH 11.5.
To check the feasibility of the second alternative, samples from the previous test were taken from the electrolytic circuit and mixed with mill pulp containing 10 pet salt. These mixtures were cyanided and the resulting pregnant solutions were precipitated in bench-scale apparatus. Gold extractions were identical to those obtained in the previous test, and barren solutions containing 0.001 oz gold per ton solution were obtained.
Scale-up experiments were conducted on a batch basis using a 6-ft by 7-ft agitator tank capable of holding 7.5 tons of pulp at 40 pct solids. Electrooxidation was accomplished with two banks of graphite electrodes used in parallel each containing 12 anodes and 11 cathodes. Tests were conducted at 40° C, 2,800-amp current, and 10-pct salt concentration in the pulp solution. Oxidized pulps were not cyanided in the pilot plant but samples were taken at 1-hr intervals and cyanided on a bench scale. Figure 16
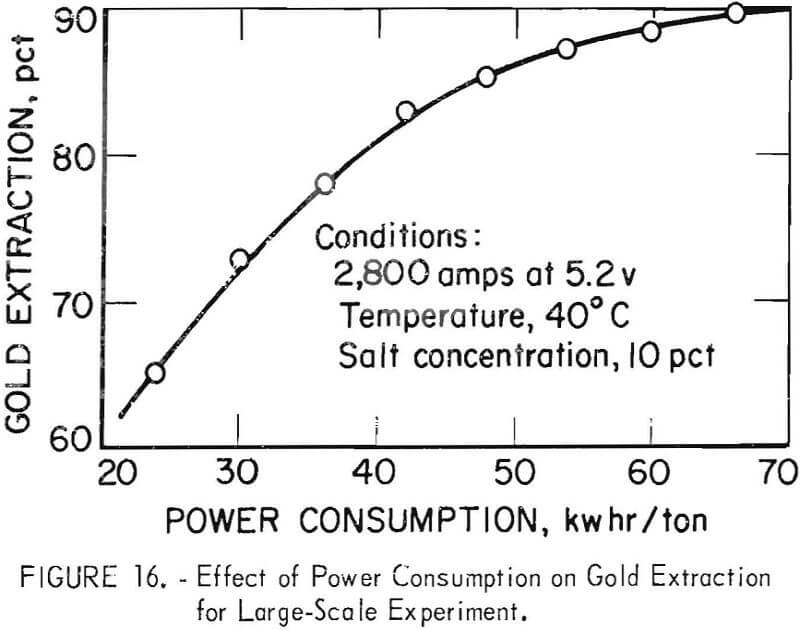
shows that gold extraction increased with electrolysis time, reaching a maximum extraction of 89.4 pct, corresponding to 22 hr of electrolysis. Data obtained from the larger experiments indicate that scale-up to commercial plants should not present any serious problems.
Since salt losses in the tailings pond can represent an important economic factor, an experiment using a salt concentration of 4 pct and the previously described PbO2-coated titanium anode-iron cathode electrode system was carried out in the 6-ft by 7-ft agitator. Operating conditions and procedures were identical to those used for the experiment with graphite electrodes. Results closely paralleled those obtained with the graphite electrodes; however, power consumption was about 20 pct greater.
Tailing pond operation and consequent salt losses were not studied; however, since approximately 80 pct of the tailing pond water is reclaimed with an ore of the type studied, about 20-pct salt loss can be anticipated once the circuit reaches a steady state.
Conclusions
Treatment of carbonaceous gold ores from north central Nevada with sodium hypochlorite or chlorine, or by electrolytic oxidation resulted in favorable gold recovery by subsequent cyanidation. The gold that is complexed by humic acid-type compounds was liberated and the adsorptive properties of the ore were passivated by the oxidation treatment. Gold extractions in the 90-pct range were obtained on several tonnage ore samples from the Carlin gold mine. Equivalent metallurgical performance, compatible with present plant practice, was obtained with sodium hypochlorite, chlorination, and electrooxidation. The choice of one of these methods is therefore dictated by economic conditions such as the cost and availability of electric power, sodium hypochlorite, and chlorine.
