For practice, a convenient material to operate on is an English silver coin, which contains 925 parts of silver to 75 parts of copper by weight. The student, therefore, knows the composition of the alloy. When dealing with alloys of unknown composition the process is more complicated (see note appended).
Method.—A given weight of the bullion is cupelled with lead sufficient to remove the copper. Any loss in cupellation is estimated by a ‘check’ assay and allowed for.
Apparatus.—The assay balance and weights, the muffle furnace and accessories, a fine watchmaker’s file.
Reagents, etc.—Sheet lead, the silver contents of which have been estimated, electrolytic copper (sheet), and test silver.
Details of the Assay.—Weigh out, as before, four lots of 1.000 each of a clean flattened silver coin, and two lots of .925 each of test silver, and two lots of .075 each of electrolytic copper, and six lots of 7 gms. sheet lead, the silver contents of which are known. Shape the lots of sheet lead into packets and arrange the packets as follows :—
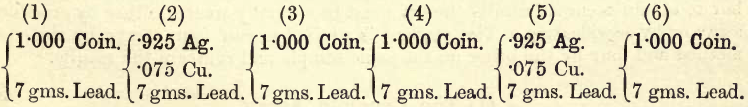
Nos. 2 and 5 are the checks ; Nos. 1, 3, 4, 6 the assays.
Mark six bullion cupels with these numbers and place them in the muffle for five minutes at a temperature slightly below a bright red. Examine, and if satisfactory, replace in two rows across the muffle,—first row, 1, 2, 3; second row, 4, 5, 6 ; the checks being in the middle. Quickly introduce the six charges.
Close the door till cupellation commences, and then open it and continue the cupellation so that ‘feathers’ just begin to form. It is rather difficult to obtain this heat, as the back row will be slightly hotter than the front; however, it may be closely approached by bringing the cupels forward in the muffle and keeping the two rows very close together, taking care, of course, that the charges do not freeze. When the blick (or coloured films) just disappears, close the doors for a few minutes and then remove the cupels, taking the usual precautions against the button spitting. When cool, clean and weigh the buttons, noting the results, and all details as to time of cupellation, temperature, etc.
Calculations.—Assume that the weights of the buttons are as follows :—
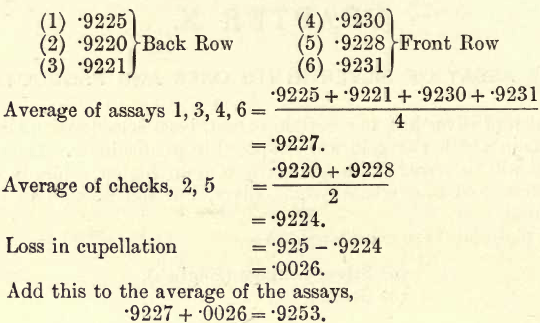
That is, the fineness of the bullion is 925.3.
Accuracy.—With good work, the cupellation loss should not be more than 6 parts per 1000, and on the average between 2 and 3 parts per 1000. This method, though not so accurate as the Volumetric method previously given, still is of a high degree of accuracy when checks are used. It further is of use in demonstrating to the student the necessity of careful cupellation when estimating silver.
Note.—If the composition of the coin were unknown, a preliminary lot of 1.000 is cupelled with about 10 gms. lead. The result is weighed, and an equal quantity of silver is taken for the check : e.g. The preliminary gives .921 Ag. Make up checks of .921 Ag and .079 Cu (or other base metal if present). Then proceed as usual with the assay and calculations.
