Toxic substances in On solutions can retard biological leaching of copper sulfide dump ores. On solutions were analyzed for metals, organics, and chloride, and showed inhibitory concentrations of aluminum, manganese, and chloride. Inhibition of bacterial activity by the solid fraction of On solution, with and without the organic constituents, was evaluated.
Materials and Methods
The most probable number (MPN) technique was used to enumerate populations of Thiobacillus ferrooxidans. Pregnant leach solution (PLS), raffinate and application (On) solution samples were diluted into Medium 64. Ore solids, from 10-15 cm depth, were diluted 1:2 into Medium 64 and vigorously mixed by vortexing for 30 seconds.
Toxicity versus contact time of the solvent extraction (SX) reagent (6% lixiviant, 94% carrier) against T. ferrooxidans was determined. Fifty mL of PLS (pH 2.13) was added to a sterile flask on a stirrer plate. Time 0 samples were taken. Fifty mL of SX reagent was added and the solution was stirred rapidly to ensure complete mixing. Samples were removed at the intervals and the T. ferrooxidans populations enumerated by MPN in Medium 64.
The total dissolved solids (TDS) in the On solution were determined according to Standard Methods. The solids were recovered from the On solution by boiling off the aqueous fraction. Half of the solids were subjected to 500°C for 4 hours in a muffle furnace to drive off residual organic compounds. All solids were heated at 110°C overnight to remove moisture. Concentrations of 50, 25, 12.5, and 6.25 g/L of solids were made in 50 mL of Medium 64.
Results and Discussion
The raffinate at this mine site received sulfuric acid additions and a ferric chloride – dewatering reagent waste stream before being recycled to the dumps as On solution. The waste stream was sometimes added on a slug basis.
The number of T. ferrooxidans in the solutions was uniformly low. No iron-oxidizing bacteria were detected in the On solution collected 8/19/92. This may have been due to a slug of waste stream.
The Minimum Inhibitory Concentrations (MIC’s) were the concentrations of soluble metals that inhibited iron oxidation by T. ferrooxidans (as judged by a lack of visible color change in the growth medium).
Concentrations of chloride, aluminum, and manganese were near toxic levels to the iron-oxidizing bacteria (F29-302) in the On solution. The resistance of this population to silver was unusually high. However, one must remember that heavy metals may be more inhibitory in combination than individually.
Organic reagents were tested individually for toxicity. No decrease in numbers of viable T. ferrooxidans in the PLS was seen even after up to 30 minutes of continuous exposure to the SX reagent (data not shown). However, up to 873 L of the SX reagent was lost each day at the plant. This could have contributed up to 46 mg/L of organic to the raffinate exiting the plant. Therefore, the effect of chronic exposure to the SX reagent was also determined (Table 4) revealing that only 16 mg/L of SX reagent was required to inhibit bacterial iron oxidation.
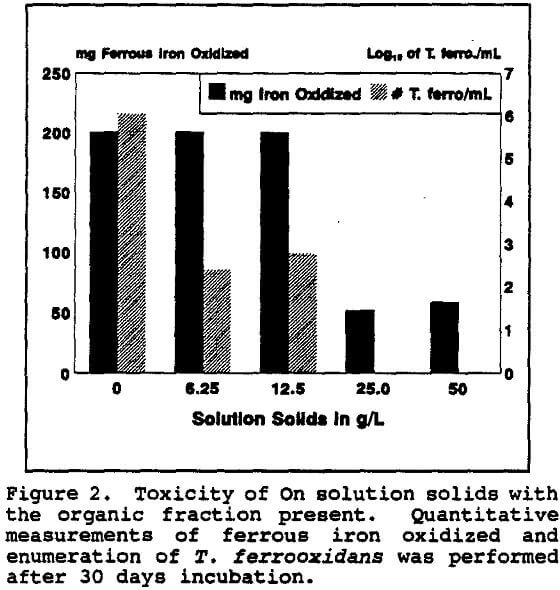
The total dissolved solids from the On solution, with and without the organic residue present, were also tested for chronic inhibition of T. ferrooxidans. The concentration of the total dissolved solids in the On solution was 41.9 g/L.
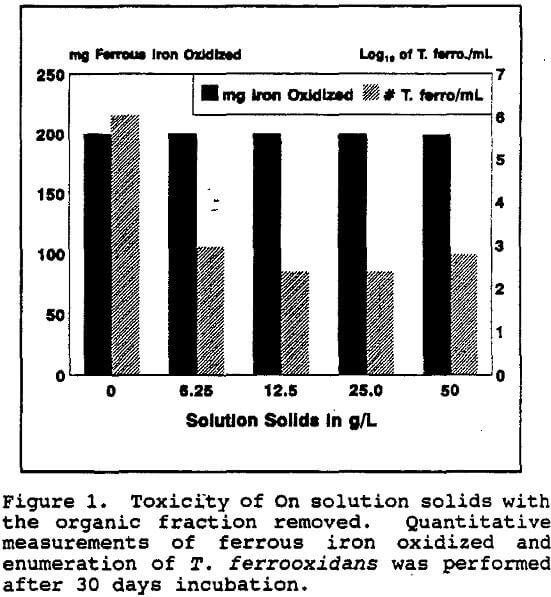
It appeared that most of the toxicity of the on solution was due to the organic fraction. Even so, the total ferrous iron oxidized was unaffected even when only low numbers of T. ferrooxidans survived. However, when all detectable T. ferrooxidans were killed, the total iron oxidation decreased precipitously as did the Eh.