Table of Contents
We demonstrated techniques for the electrowinning of tungsten directly from scheelite concentrate. The major problem with direct electrowinning is the accumulation of lime (CaO) in the electrolyte. Scheelite mineral concentrates usually contain 60 to 70 weight-percent WO3 and about 20 weight-percent CaO.
The results of the CaO buildup are decreased metal purity, increased electrolyte viscosity, and decreased metal adherence to the cathode. The effects of CaO accumulation were neutralized by periodic additions of boric oxide (B2O3) to the electrolyte, but relief was only temporary. Electrowinning from commercial-grade tungstic oxide (WO3) was conducted for a prolonged period without degradation of the products or significant buildup of impurities in the electrolyte. The quantity of electrolyte per pound of tungsten recovered was 1.33 pounds. In direct electrowinning from scheelite, 3.93 pounds of electrolyte were required per pound of tungsten recovered.
A technique for the preelectrolysis elimination of the CaO from the scheelite concentrates would be desirable. One method that appears feasible is the decomposition of scheelite in hot HCl, represented by the following reaction:
CaWO4 + 2HCl → H2WO4 + CaCl2……………………………………………..(1)
The product is crude tungstic acid containing acid-insoluble material from the concentrate, mainly silicate minerals.
Another possibly feasible method is the high-temperature, two-phase separation technique, based on the reaction with sodium metasilicate at 1,080° C in the presence of molten NaCl:
CaWO4 + 2Na2SiO3 → Na2WO4 + Na3CaSi2O6……………………………………..(2)
The most efficient separation is obtained when 2 moles of Na2SiO3 are used per mole of scheelite. The sodium tungstate (Na2WO4) is recovered in a molten NaCl phase which is immiscible with the silicate phase. Optimum extractions are 99 percent of the WO3 in the halide phase and 88 percent of the CaO in the silicate phase. Electrodeposition of either tungsten metal or tungsten carbide (WC) from the Na2WO4-NaCl melt may be accomplished, depending upon the chemicals added to the melt to form the electrolyte.
The objective of this study was to investigate the hydrochloric acid digestion and high-temperature, two-phase separation techniques for the electrolytic preparation of tungsten. The results of the processes are evaluated and compared with those of direct electrowinning of tungsten from scheelite concentrate.
Experimental Materials and Equipment
The analysis of California scheelite concentrate used as the tungsten source material is as follows, in weight-percent:
WO3……………………………………………………………..73.51
CaO………………………………………………………………20.14
Fe2O3……………………………………………………………1.27
SiO2………………………………………………………………1.20
Al2O3…………………………………………………………. .20
MoO3…………………………………………………………. .40
The concentrate had the following particle size distribution, in weight- percent: plus 65 mesh, 2 percent; minus 65 plus 100 mesh, 8 percent; minus 100 plus 200 mesh, 24 percent; and minus 200 mesh, 66 percent.
Technical-grade salts were used in the electrolytes and in the two-phase separation. Chemically pure-grade hydrochloric acid was used in the leaching experiments. The two-phase separation and most of the electrolysis experiments were performed in 3-inch-inside-diameter by 8-inch-high graphite crucibles. Electrolysis using crude oxide feed was performed in a graphite crucible of 5-inch inside diameter and 11 inches in depth. The crucible was the cathode, and a centrally positioned 1.5-inch-diameter graphite rod was the anode. The crucible was heated to operating temperatures of 1,000° to 1,080° C in a globar furnace.
Experimental Results
Acid Digestion Process
HCl Digestion of Scheelite Concentrate
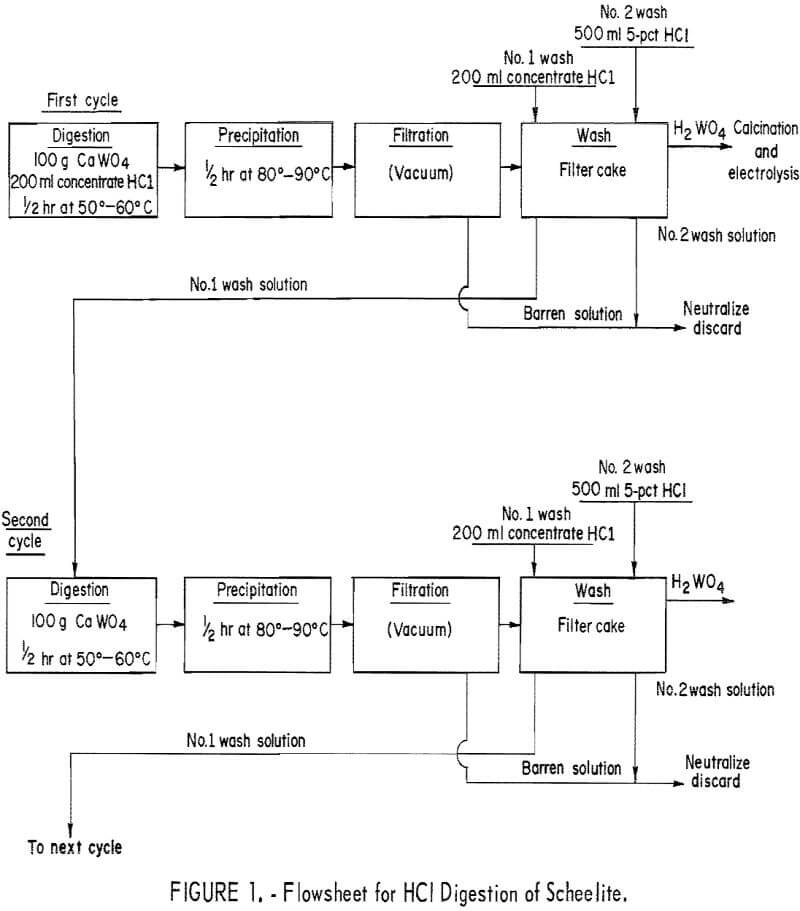
Conventional acid decomposition of scheelite requires 1.5 to 2.0 parts of concentrated HCl per part scheelite. This technique produces a tungstic acid slurry, which can be filtered and washed free of soluble impurities. The tungstic acid is then dissolved in ammonia or sodium hydroxide to form ammonium paratungstate or sodium tungstate. Our experiments were designed to determine the most efficient method to convert the scheelite concentrate to an oxide cell feed suitable for electrowinning. The following operating variables were studied: Ratio of HCl to scheelite, digestion time, digestion and precipitation temperature, and procedures for washing the tungstic acid precipitate. The digestions were performed in an Erlenmeyer flask with a reflux condenser. The flask was charged with 100 grams of scheelite concentrate and 200 ml of concentrated HCl. The charge was stirred with a magnetic stirrer and heated on an electric hotplate.
The procedure diagramed in figure 1 was determined to be the most efficient method for preparing a crude tungstic oxide. The procedure required about 2 ml of concentrated HCl per gram of scheelite. Digestion and precipitation required only 30 minutes each. The first wash solution of concentrated HCl was recycled to leach the next 100-gram charge of scheelite. The digestion temperature was 50° to 60° C and precipitation temperature was 80° to 90° C. Within a few minutes after digestion had begun, the yellow tungstic acid started to precipitate. This tendency of tungstic acid (H2WO4) to precipitate from an acid solution eliminates the possibility of using filtration to remove the acid-insoluble silicate minerals. Analysis of the tungstic acid precipitate showed that two washings were adequate to remove soluble impurities. The acid solutions could be neutralized with limestone (CaCO3) or treated by other means to meet local pollution standards prior to discarding.
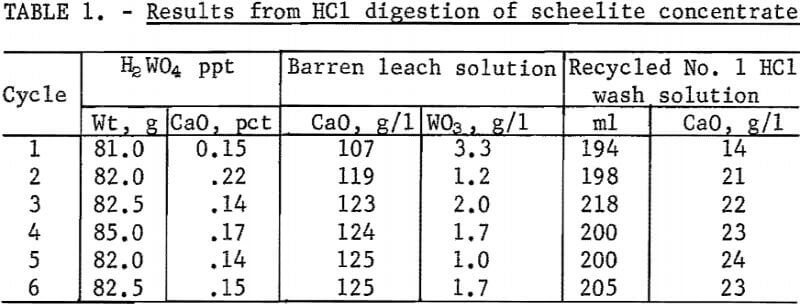
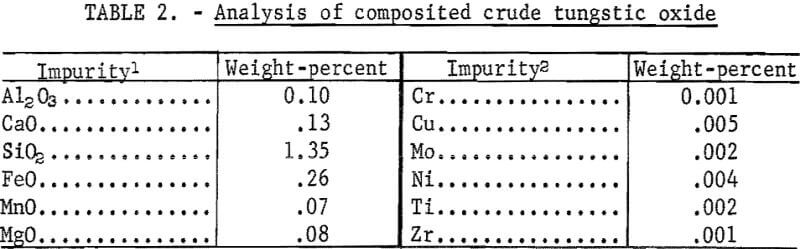
The results of 6 successive leach cycles are shown in table 1. A steady state was reached after the second cycle as indicated in table 1. Analysis of the oxide formed by calcining the tungstic acid from the 6 cycles is shown in table 2. Loss on ignition was approximately 10 percent. Over 99 percent of the tungsten in the scheelite was recovered in the product which contained 98-percent WO3. The technique was scaled up to treat 300-gram batches of scheelite concentrate. Seven kilograms of oxide were prepared for use as cell feed. The analysis of the 7-kilogram composite of tungstic oxide was almost identical to that shown in table 2.
Electrolysis of Crude Oxide
The procedure for electrolysis was a modification of the technique reported previously for electrowinning of tungsten from WO3. The original 5 kilograms of electrolyte contained, in weight-percent: WO3, 20; sodium pyrophosphate (Na4P2O7), 60; sodium tetraborate (Na2B4O7), 4; sodium chloride (NaCl), 14; and calcium fluoride (CaF2), 2. The electrolyte was heated to 1,000° C by the external furnace and electrolysis was initiated, using the crucible as the cathode. By mechanically feeding tungstic oxide, the WO3 content of the electrolyte was maintained between 10 and 20 percent.
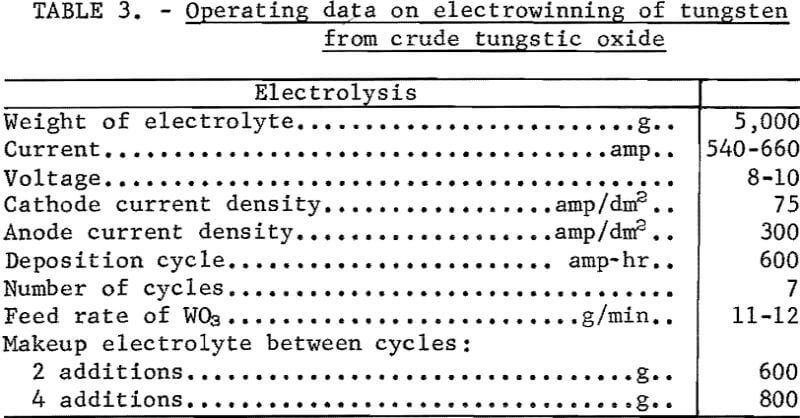
The electrolysis current of 540 to 600 amperes was sufficient to maintain the bath temperature between 1,000° and 1,100° C, and no external heating was required. At the completion of 600 ampere-hours of electrolysis, the feeder was stopped, the anode was removed, and the metal was recovered from the cell with a graphite scraper. Makeup electrolyte was added to the cell, the anode repositioned, the feeder started, and the electrolysis cycle repeated. The metal deposits were cleaned of adhering electrolyte by leaching, first in hot 5-percent HCl, and then in a 2-percent NaOH solution. The operating data and results for a cyclic experiment are shown in tables 3 and 4. The average cathode current efficiency was 89.5 percent, and 3.9 kilowatt-hours of power was consumed per pound of tungsten recovered.
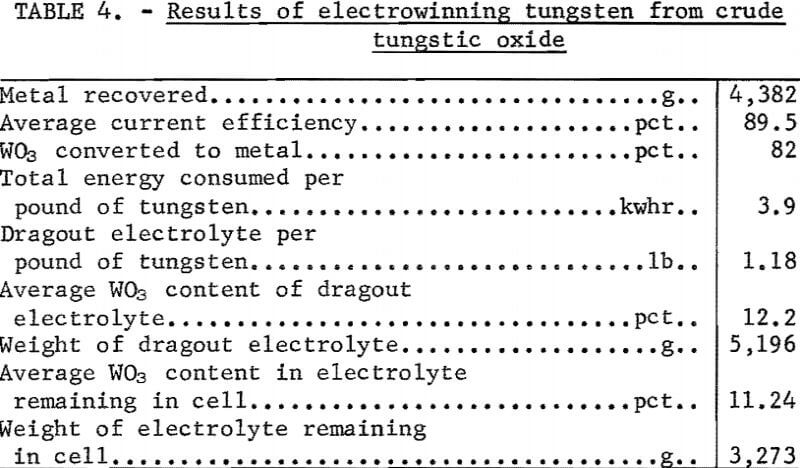
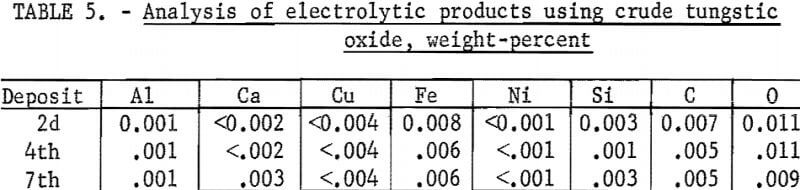
The analyses of the products are shown in table 5. Metal purity was in excess of 99.9 percent and comparable to metal electrowon from pure WO3. The overall tungsten recovery from the WO3 cell feed was 93.5 percent, excluding WO3 in the original and the final electrolyte. WO3, in the dragout electrolyte, could be reclaimed and recycled as cell feed. The major losses of materials, which were from dusting and fuming, amounted to 2.3-percent WO3 and 1.1-percent electrolyte salts.
Two-Phase Separation Sequence
Extraction of WO3 from Scheelite Concentrate
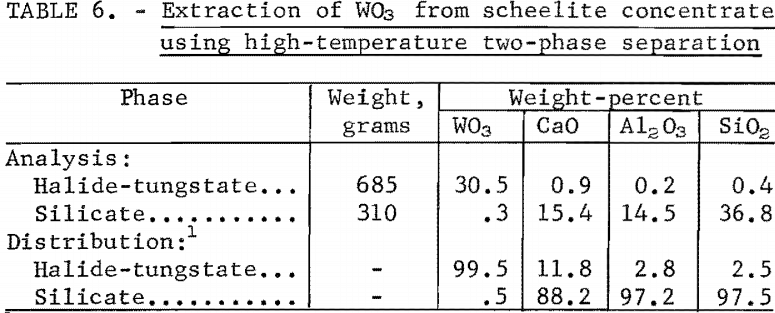
The high-temperature, two-phase separation sequence is based on the extraction of WO3 in an immiscible two-phase molten salt system. Scheelite concentrate was dissolved in a molten halide-silicate mixture at 1,070° C and held for 1 hour. Tungstic oxide (WO3) was converted to Na2WO4 and transferred to the upper halide phase; calcium oxide was retained in the lower silicate phase. The two liquid phases were separated by decantation.
WO3 extraction of 99.5 percent and a separation factor of 1,550 were obtained with a melt of the following composition:
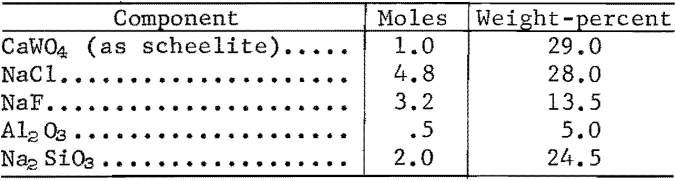
The analyses of the two phases and percentage distribution of oxide constituents are shown in table 6.
Electrolysis of Halide-Tungstate Melt
Electrolysis of the halide-tungstate melt as obtained from the two-phase separation did not result in the deposition of any metallic product. Additions of phosphate, borate, or alkali aluminum fluoride to the melt were required to form a satisfactory electrolyte. The best results were obtained when 2.0 moles of NaPO3 and 1.5 moles of B2O3 were added per mole of WO3 in the melt.
Electrolysis was conducted at 1,000° C using external heating. The crucible was the cathode and a centrally positioned 1-inch graphite rod was the anode. The applied current was 100 amperes, the cell potential was 4.5 to 5.0 volts, and the cathode current density 35 ampere/square decimeter. After 150 ampere-hours of electrolysis, the electrolyte was decanted from the crucible. The deposit was then removed from the crucible by soaking in water and scraping.
The deposit weighed 140 grams, representing a recovery of 83 percent of the tungsten in the scheelite. The spent electrolyte analyzed 2.7 percent WO3 and contained 10.5 percent of the original tungsten value. The current efficiency was 81.5 percent, and 5 kilowatt-hours were required per pound of tungsten deposited. The product was of high purity and contained the following impurities, in weight-percent: C, 0.023; O, 0.008; Fe, 0.005; Al, <0.001; Ca <0.002; and Si, <<0.001.
Comparison of Electrowinning Techniques
Direct electrowinning techniques were described in detail in a previous Bureau of Mines publication. The results of the most efficient technique were selected for comparison with those of the electrolysis of crude WO3 prepared by the acid digestion process and two-phase separation sequence. Results, material, and energy requirements for producing tungsten by the three methods are shown in table 7. The analyses of the three products are shown in table 8.
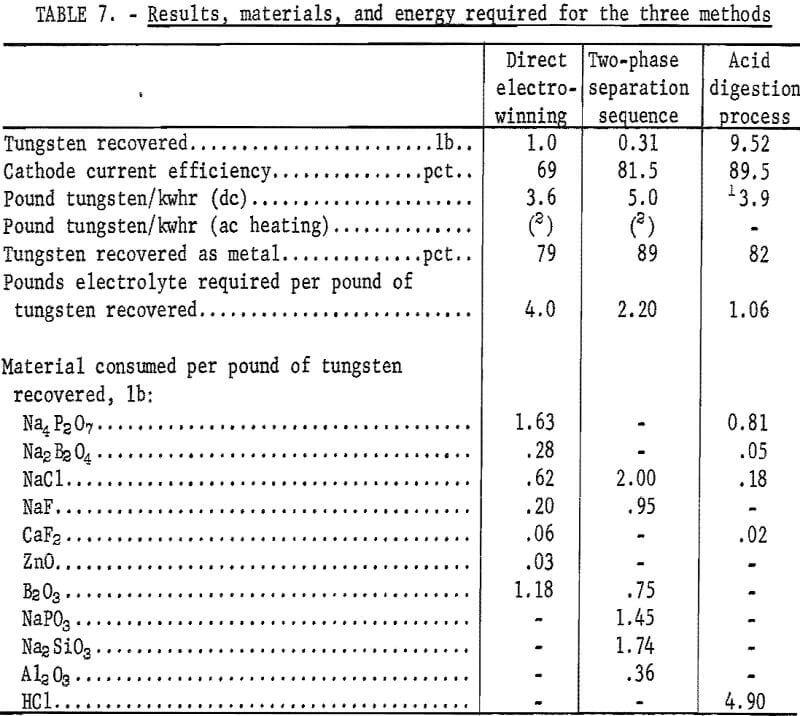
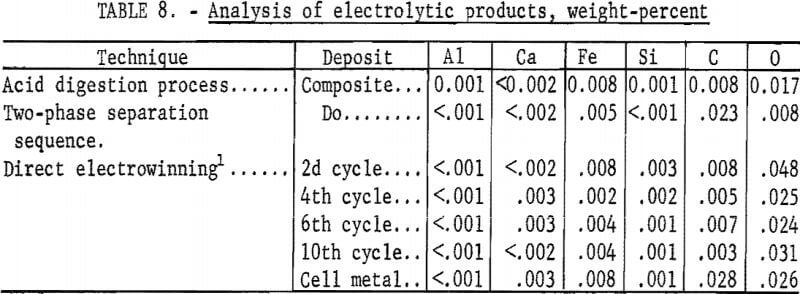
Metal of 99.9-percent purity was electrowon, using each of the techniques. The extent of conversion of tungsten to metal was similar, varying from 79 to 83 percent. Most of the tungsten loss was in the dragout electrolyte and could be recovered by the hydrometallurgical processing of this material. The anode carbon consumption was 0.22 pound per pound of tungsten recovered.
The acid digestion process has the following advantages over the other two methods:
- Prolonged electrowinning operation.
- Lower rate of impurity buildup in the electrolyte.
- Less electrolyte reagents consumed per pound of tungsten recovered.
- Higher cathode current efficiency.
- Less total electrical energy required per pound of tungsten recovered.
Disadvantages of the HCl-digestion process are the 5 pounds of acid required to produce 1 pound of metal and the additional processing equipment and labor needed.
The two-phase separation sequence for recovering tungsten from scheelite is mainly of academic interest. The technique is more applicable to the treatment of acid-insoluble wolframite ores and possibly scheelite ores containing a high percentage of iron. The two-phase separation sequence consumes 7.25 pounds of reagents per pound of tungsten produced.
The direct electrolysis of scheelite is an intermittent operation. Lime and other associated impurities from the scheelite accumulate in the electrolyte. This accumulation results in a degradation of the product and decreases metal adherence to the cathode. Periodic additions of boric oxide are required to neutralize the effects of the CaO buildup. This is only a temporary relief measure because synthetic apatite (Ca5(PO4)3F) forms in the electrolyte, and the resulting scum must be removed periodically.
Conclusions
The most efficient electrolytic method for preparing tungsten from scheelite was to decompose the mineral with hot, concentrated HCl to prepare a crude WO3 cell feed. Metal of 99.9-percent purity was won from the prepared oxide. Metal deposition in the seventh cycle showed no decrease in purity. This technique requires about 4 kilowatt-hours of electrical power, 5.0 pounds of concentrated HCl, and 1.0 pound of electrolyte reagent per pound of tungsten recovered.
Metal of 99.9-percent purity was recovered with both the high-temperature, two-phase separation sequence and direct electrolysis. The two-phase separation sequence required over 8 kilowatt-hours of electrical power and 7.25 pounds of reagents per pound of tungsten recovered. The direct electrolysis required about 7 kilowatt-hours and 4.0 pounds of reagents per pound of tungsten recovered.
