Composite diaphragms were constructed and used for the electrowinning of Ti from TiCl4 to eliminate some of the problems associated with diaphragm failure. High-purity titanium (Ti) was previously electrowon from titanium tetrachloride (TiCl4) fed into a molten electrolyte (520° C) composed of lithium chloride, potassium chloride, and titanium dichloride (LiCl-KCl-TiCl2). Two papers describing the work were published. Composite type diaphragms were patented in 1897. Leone and coworkers described a cell equipped with commercially available alumina (Al2O3) diaphragms of an approximate composition of 86 percent Al2O3 and 12 percent silica (SiO2). Couch and coworkers described the operation of cells scaled up to 100 amperes and operated on a continuous basis using these commercially available diaphragms. They also evaluate other commercially available refractory materials as diaphragms.
Materials found suitable as diaphragms were aluminum oxide (Al2O3), silicon dioxide (SiO2), magnesium oxide (MgO), zirconium oxide (ZrO2), beryllium oxide (BeO), thorium oxide (ThO2), boron nitride (BN), porcelain and Al2O3-12 pct SiO2. All materials tested slowly reacted with the LiCl-KCl-TiCl2 electrolyte and caused titanium to deposit in the diaphragm material. The amount of titanium present was increased by material fineness, immersion and operational time, temperature, and current density. Electrically conducting materials, such as graphite, acted as a bipolar electrode and would not operate satisfactorily as a diaphragm. As Ti content increased, the ceramic diaphragms became better electrical conductors and less effective as diaphragms.
Two factors that were identified as affecting diaphragm life were the temperature of the electrolyte and the anode diaphragm current density. When operating temperatures were raised much above 520° C, the useful life of the anode diaphragm was reduced. Useful life of the diaphragm increased as the diaphragm current density decreased. This diaphragm current density was based on the amount of current flowing through the diaphragm. For example, life of a diaphragm at 120 amp per ft² was 22,000 ampere-hours, and at 60 amp per ft², 66,000 ampere-hours. In addition to the limitation of a low current capacity, the ceramic diaphragms had a limited life because they were fragile and subject to breakage and spalling. The composite diaphragms studied in the present investigation were less subject to mechanical breakage.
Equipment and Procedure
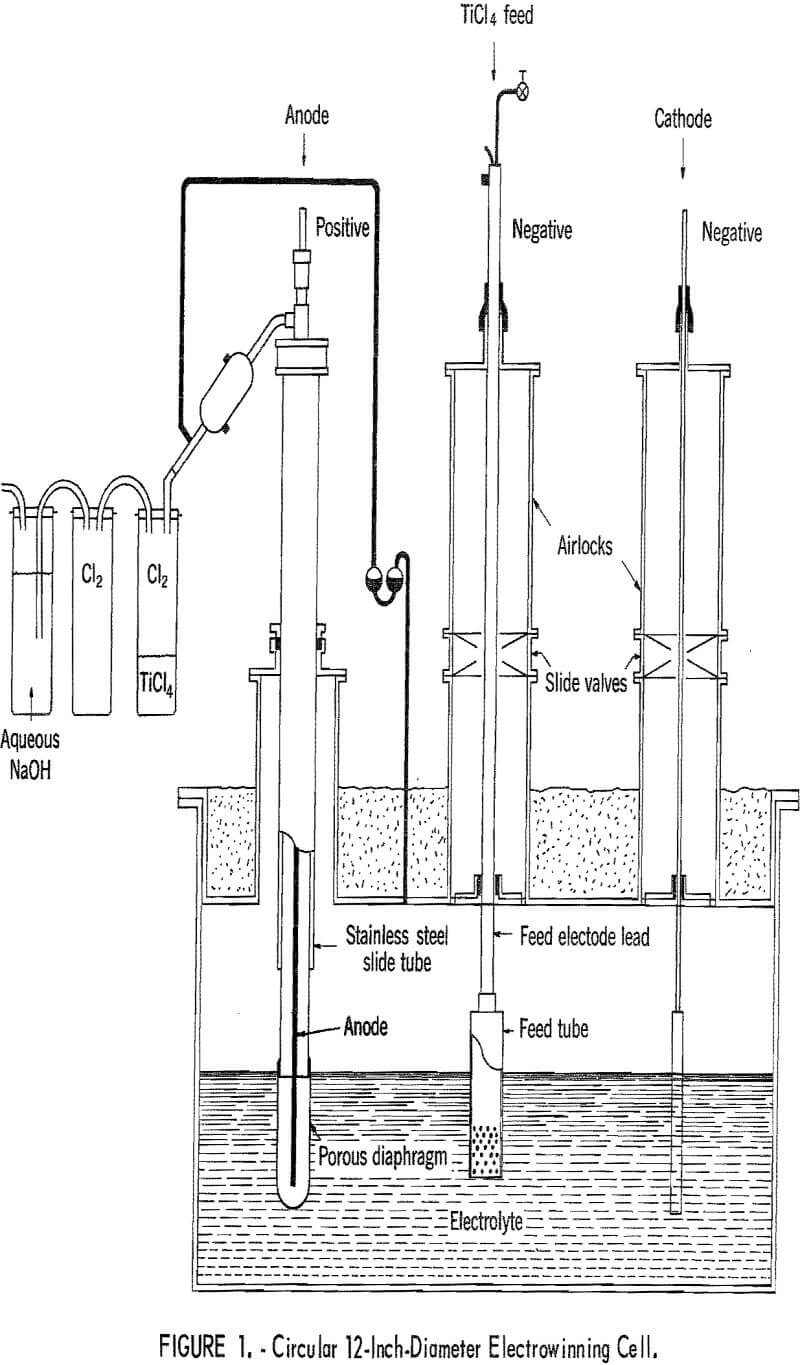
Circular 12-Inch-Diameter Cell
The same 12-inch-diameter electrolysis cell and procedure of operation described by Couch and Leone was used in the composite diaphragms investigation. A schematic drawing of the cell and accessories is shown in figure 1. An inert atmosphere was maintained in the cell, which contained approximately 60 pounds of electrolyte. The electrolyte was prepared by pumping TiCl4 through a water-cooled feed tube into the molten LiCl-KCl. Two faradays of current were passed through the cell for each mole of TiCl4 feed until the desirable 5 percent Ti++ was obtained. Then a cathode was introduced and two faradays of electric current continued on the feed tube. At the same time, two faradays were applied to a deposition cathode per mole of TiCl4 to give a crystalline deposit of titanium metal on the cathode. The porous diaphragm permitted the flow of ions, but retarded the flow of liquids and gases through the diaphragm. Anodically discharged chlorine was conducted through an enclosed passage for recovery outside of the cell and the titanium deposit was removed through an airlock for processing.
The electrodeposition cathode current efficiency was based on the weight of titanium deposited in proportion to the theoretical weight that should be deposited, assuming a valence of four for titanium. Anode efficiency was based on the amount of chlorine produced at the anode and was not corrected for the current used in the production of TiCl4 at the anode.
To utilize and incorporate the desirable properties of each of two or more component materials for the diaphragm structure, the composite diaphragms represented by figures 2 and 3 were constructed and tested. Composite diaphragms depicted by figure 2 utilized one of the proven solid diaphragm materials or an Al2O3 refractory cement coating as the inner dielectric
structure proximate to the anolyte and a tightly fitting porous metal or other material for the outer structure proximate to the catholyte. Composite diaphragms that utilized both solid and loose materials in their structure are depicted by figure 3, in which the loose material is contained between solid inner and outer structures. The size and form of the cylindrical composite diaphragm was determined by the characteristics of the structural materials; in general, the dimensions varied from 2 to 3-½ inches OD, 8 to 12 inches in length, and with wall thicknesses of 1/16 to ¾ inch.
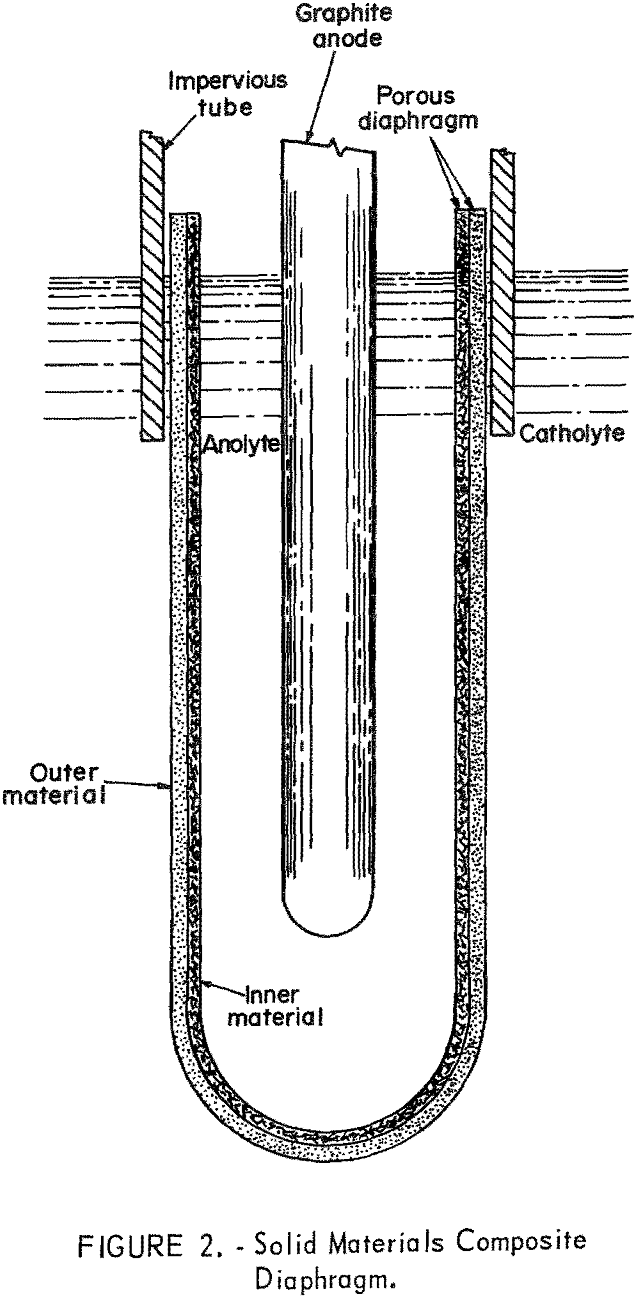
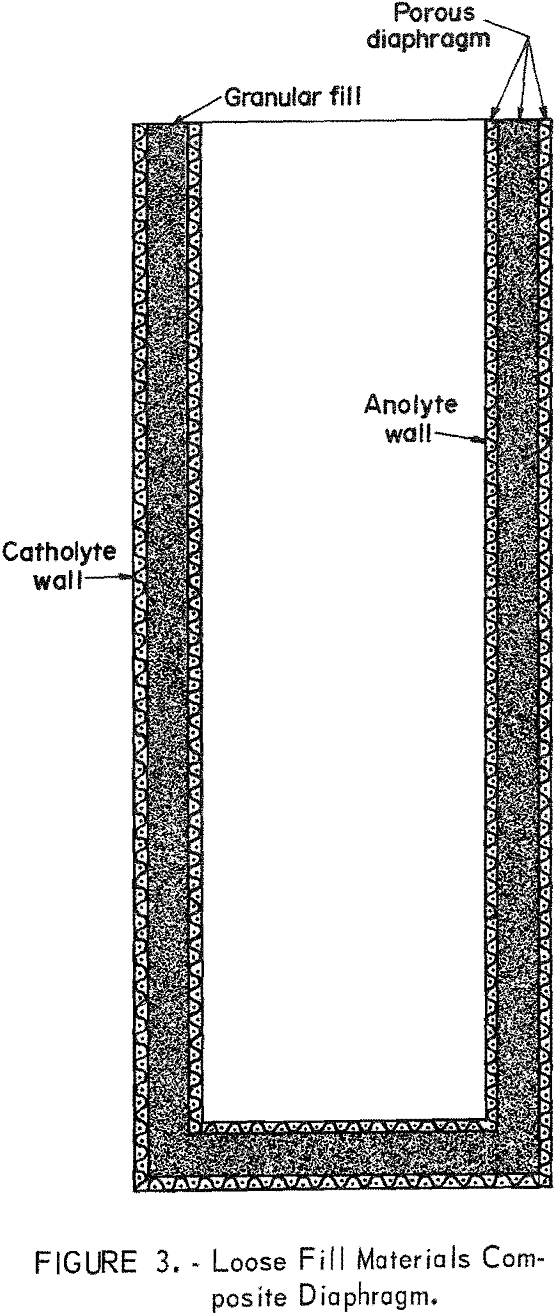
Solid Materials Composite Diaphragms
Shown in table 1 are the diaphragm descriptions and test data on the solid materials composite diaphragms that were made and tested.
Diaphragm 1 was a 120-mesh nickel screen basket with an inner surface coating of Aremco 505-Ceramacast alumina cement which was heat-cured before using. The porous diaphragm length subject to electrolysis was approximately 7 inches and the upper remaining length of the diaphragm, which was overlapped by the open end of the impervious gas tube, formed the molten-salt seal. After 30 hours of continuous electrolysis at a diaphragm current density of 70 amp per ft², a 1-inch-long split developed in the wall. The cement was still tightly adherent to the screen, however, and a 43-percent titanium replacement of alumina occurred. Workability of the composite diaphragm was shown by the 70-percent average chlorine efficiency.
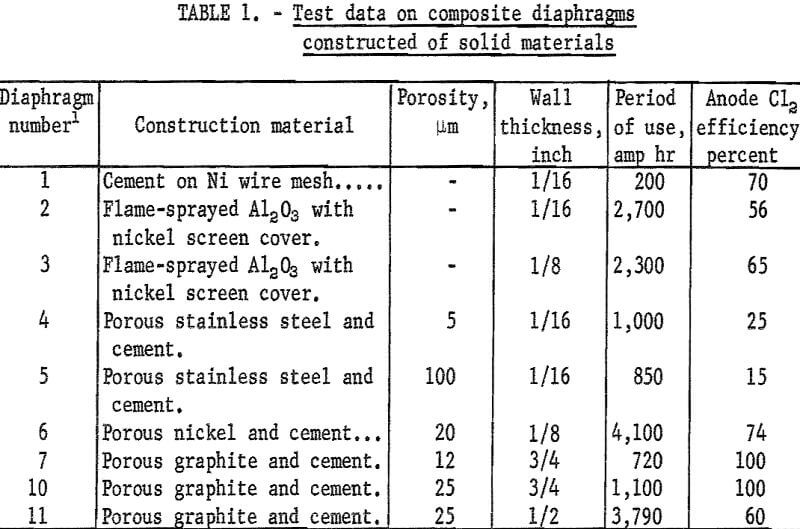
Diaphragms prepared by flame spraying alumina powder onto a brass mandrel were shown in previous studies (1) to be equal in performance to the commercially available alumina diaphragm. Two of the flame-sprayed diaphragms were made into composite diaphragms and strengthened by covering the outer surface with closely fitting 20-mesh screen (diaphragms 2 and 3, table 1). One test ended after 2,700 ampere-hours because of K2TiCl6 formation which blocked effluent gas passage. The low average chlorine efficiency of 56 percent resulted from the restricted and blocked flow of chlorine. The used diaphragm showed no deterioration except at electrolyte level. A similar test ended with breakage of the Vycor gas tube on completion of 2,300 ampere-hours, with an average chlorine efficiency of 65 percent. Although complete evaluation of the composite flame-sprayed diaphragms could not be made because of operational irregularities, the indication was that they were as durable as the commercial alumina diaphragm.
Two porous stainless steel diaphragms (diaphragms 4 and 5, table 1) were made into composite diaphragms by covering the inner walls with cement. The porosity rating of the diaphragms was 5 and 100 µm, respectively. Test series on the diaphragm of 5 µm porosity was terminated after 1,000 ampere-hours because of low average chlorine efficiency (25 percent). It was found that an incomplete seal existed between gas tube and diaphragm, allowing the escape of chlorine and extensive corrosion of diaphragm at the seal area. Test series on diaphragm 5, 100 µm porosity, was terminated after 850 ampere-hours because of the low average chlorine efficiency (15 percent). The greater porosity enabled the interflow of gases causing corrosion of diaphragm and supporting nickel wires. These tests indicate that the composite stainless steel diaphragms are operative, but require stringent precautions to avoid corrosion from chlorine.
Diaphragm 6 was porous nickel with a pore size of 20 µm. Its wall thickness was twice that of the stainless steel diaphragms, but of the same outside diameter and length. To obtain an inner ceramic coating of greater porosity an equal portion of Alundum-60 granules was used with the cement. The series was terminated for evaluation on completion of 17 tests totaling 4,100 ampere-hours. Chlorine efficiencies averaged 74 percent. The used diaphragm was sectioned for examination and showed the coating to be intact and adherent, with no diaphragm corrosion or other deleterious effects. Service-ability of this type of composite porous metal diaphragm appears to be good.
Graphite composite diaphragm 7 was made by machining porous graphite with a rated porosity of 12 µm into a thimble having an area subject to electrolysis approximately the same as previous diaphragms and coating the inner surface with a 1/16-inch thickness of cement. A total of 720 ampere-hours was completed in the first 5 tests. TiCl4 feed was then discontinued and the series continued for 7 more tests for a total of 1,560 ampere-hours. Current densities on outer and inner diaphragm surfaces were 45 and 60 amp per ft², respectively. The procedure of stripping titanium from the electrolyte in the latter tests was to determine if a bipolar condition occurred on the graphite diaphragm. The titanium valence remained constant throughout the tests, denoting no oxidation or bipolar effect. Chlorine recovery for the series was practically 100 percent, which also shows there was no bipolar effects. Cathode efficiency averaged 85 percent and metal hardness was 75 Bhn. Tests were terminated for evaluation of used diaphragm. There was no deterioration of either the graphite or the cement coating.
Composite graphite diaphragms 8 and 9 were made from the same graphite stock and cement as diaphragm 7. Diaphragm 7 had a ¾-inch wall, diaphragm 8 had a ½-inch wall, and diaphragm 9 had a 3/16-inch wall. The thinner walls were to allow an increased current flow and current density. Testing of diaphragm 8 ended in the first test when the anode broke. Since appearance of diaphragm 8 was good, it was leached, dried, and retested; however, it fractured on immersion in the molten electrolyte, evidently from heat stresses or trapped salt. With the thinner walled diaphragm 9, no effluent was obtained at 20 amperes current. To overcome intermixing, the current was increased to 40, then 60 amperes in the next two tests before a satisfactory effluent of TiCl4, then chlorine, was obtained. The inner surface current density of diaphragm operating at 60 amperes was 195 amp per ft², which resulted in diaphragm rupture soon after the chlorine flow started.
Composite graphite diaphragms were also made from 25-µm-porosity graphite stock. These diaphragms (10 and 11) had approximately the same dimensions as those of the preceding diaphragms. The ¾-inch walled diaphragm 10 ruptured on completion of 1,100 ampere-hours. Chlorine recovery of practically 100 percent was obtained in the series.
The ½-inch-thick walled graphite diaphragm 11 operated efficiently for 1,450 ampere-hours with an inner surface current density of 60 amp per ft². The current density was then increased to 130 amp per ft² and the operation continued efficiently through 2,200 ampere-hours when the current density was again increased to 195 amp per ft², where operation continued for 140 ampere-hours more when diaphragm rupture resulted.
A piece of standard (CS-grade) graphite was used to make composite diaphragm 12 with the same dimensions as those used previously, except for the 5/16-inch wall. Even at a diaphragm current density of 300 amp per ft², no effluent was produced in the tests. The used diaphragm and cement coating showed no deterioration and apparently failure of the standard graphite to function as a diaphragm was because of low porosity and bipolar effects.
The porous graphite serviceability as a composite diaphragm is not much better than that of alumina and some of the other refractory porous diaphragm materials and is subject to the same limiting factor of low-current density.
Loose Fill Materials Composite Diaphragms
Data from six loose fill materials composite diaphragms are shown in table 2. Alumina granules, silica sand, and boron nitride fibers were the loose materials used to fill the diaphragms. To retain the loose granules, the support contiguous to the catholyte was made of 80-mesh nickel screen. A 30-µm-porosity alumina diaphragm was used for the liner of diaphragm 13, and the liner for diaphragm 14 was a 20-mesh nickel screen coated with cement. To attain a greater porosity the screens of diaphragms 15 and 16 liners were coated with a mixture of 2 parts Alundum-60 (approximately 60 mesh) granules and one of cement.
Using a 15-ampere current, 18 continuous 24-hour tests were completed for a total of 6,000 ampere-hours when the diaphragm 13 series was terminated. The anode efficiencies of practically 100 percent obtained early in the series began decreasing and were down to 50 percent at termination. The decreasing efficiency was from backflow of chlorine through a widening space above the diaphragm fill level. With progressive use the loose material settled, resulting in a gap at the top of the diaphragm. The used diaphragm was in good condition except for a slight corrosion proximate to the space where the backflow of chlorine occurred. The 23-percent Ti replacement of the alumina liner material was normal for this period of electrolysis; however, a highly favorable result was the low 0.95-percent Ti replacement for the Alundum-60 fill. Liner current density was 65 amp per ft² and the average cathode efficiencies (55 percent) were good, considering the loss of chlorine into the cell. Average product quality was 68 Bhn. This type composite diaphragm appears to have good serviceability.
To gain strength, a cement-coated nickel screen liner was used in diaphragm 14. Seventeen continuous tests of 24 hours each were completed for a total of 12,600 ampere-hours. Using a 15-ampere current the average chlorine efficiencies of the first four tests was 70 percent. The gas tube broke at this time, but remained sufficiently operative, because the nickel wires used as supports held the broken tube firmly in place. Since the prime objective was to test the liner the series was continued and current was increased to counteract the chlorine loss as the crack widened. When the series was terminated the current was 75 amperes and the current density on the liner was 300 amp per ft². Even though the conditions imposed on the diaphragm were severe, no deterioration had occurred on the liner or other components of the diaphragm.
Diaphragm 15 liner coating was made more permeable by using a mixture of two parts Alundum-60 granules to one part cement. Initial tests showed that the diaphragm was too permeable. The current was increased to 100 amperes to counteract the backflow of chlorine. Although a considerable flow of effluent chlorine and TiCl4 was obtained, there was sufficient backflow of chlorine to corrode a hole in the screen cover. The inner basket showed no sign of deterioration and Ti replacement of the Alundum-60 fill was only 0.2 percent.
Diaphragm 16 was made less porous than diaphragm 15 by using Alundum-120 granules for the fill and coating the cover with a mixture of two parts Alundum-60 granules and one part cement. The cover coating also served to protect it from corrosion by the backflow of chlorine. Thirty-two tests at 30 amperes were made for a total of 7,500 ampere-hours. As had occurred in the diaphragm 14 series, the gas tube cracked on completion of test 15, and as before, the crack was held sufficiently tight to allow continuation of the series. Both the chlorine and cathode efficiencies for the first 15 tests averaged 81 percent. Efficiencies for the series averaged 71 percent for the chlorine and 67 percent for the cathode. Product quality was 75 Bhn. Titanium replacement in the Alundum-120 fill was 0.6 percent. There was no deterioration of the diaphragm. Good serviceability can be expected from this type of diaphragm.
Diaphragms 17 and 18 were both filled with minus 80- plus 100-mesh silica sand. For diaphragm 17 the 20-mesh nickel screen used for both the cover and the liner was covered with a coating of equal parts of Alundum-120 granules and cement, whereas the 20-mesh nickel screen wire and 10-mesh nickel screen liner of diaphragm 18 were coated with cement.
Only nine 8-hour tests at 30 amperes were completed on diaphragm 17. Chlorine efficiencies of practically 100 percent were obtained in the first 5 tests when the gas tube broke. As tests were continued efficiency decreased, and was 30 percent on termination. Ti replacement of silica was 4 percent. Though evaluation was restricted because of gas tube breakage, the indication is that use of low-cost silica sand fill in diaphragm construction is operative and feasible.
Nineteen tests at 30 amperes totaling 4,400 ampere-hours were made on diaphragm 18. Operational irregularity resulted from a leaky slide valve resulting in atmospheric contamination of electrolyte and gradual blockage of gas passage. The 90-percent chlorine efficiencies obtained in the first 4 tests began decreasing and averaged 65 percent for the series. Ti replacement in the sand fill was 4 percent. Although the operation was made under adverse conditions, the diaphragm showed no deterioration.
Cement was used to coat the 10-mesh nickel screen used for both the cover and liner of diaphragm 19. The fill was a ½-inch thickness of tightly packed BN (boron nitride) fibers. Though packed tightly, the BN fill was still too permeable. Fifteen tests at 45 amperes were completed for a total of 5,000 ampere-hours. Chlorine efficiencies were from 63 to 83 percent. There was no sign of corrosion or deterioration and the BN fibers retained their form. Ti replacement in the BN fibers was 1.0 percent. This replacement was low considering the fineness of the fibers and would undoubtedly be lower in a granular BN fill. The workability was good, but the cost high.
Rectangular Cell
Shown in figure 4 is a schematic drawing of the electrowinning cell constructed with a central compartment to accept a 1-foot-square, flat diaphragm of loose material, and a gate. The cell was designed to maintain an inert atmosphere and eliminate such operational irregularities as broken gas tubes, K2TiCl6 blockage of gas passage, and diaphragm corrosion from backflow of chlorine. Contiguous to the loose material diaphragm side of the center compartment was the anolyte compartment accommodating the dense graphite anode and having an effluent discharge port through the top. The catholyte compartment was contiguous to the gate side of the center compartment and contained the TiCl4 feed tube and two cathodes. The gate prevented intermixing of anolyte and catholyte when diaphragm was withdrawn into airlock compartment for servicing.
The 500-pound charge of LiCl-KCl-TiCl2 electrolyte contained 5 percent soluble Ti, and during operation TiCl4 was fed continuously. It was found that when the 520° C operating temperature was reached the binding cement used to seal the diaphragm compartment to the cell walls had become pervious
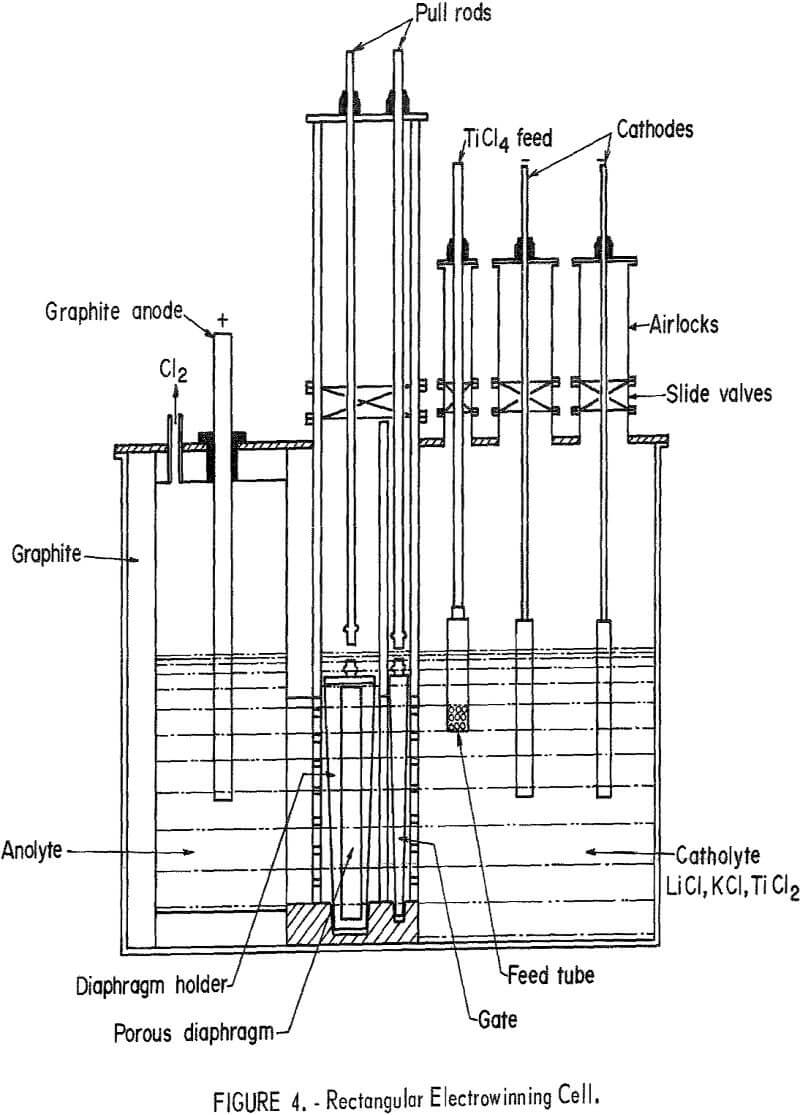
and a positive seal between the compartments was not achieved. Rather than rebuild the cell, it was decided to attempt operation under this adverse condition in order to determine its operational worth and also endeavor to obtain an evaluation of the flat composite diaphragm with a silica fill similar to those used in diaphragms 17 and 18 in table 2.
The cell was operated continuously for 84 hours, except for three shutdowns for cell repairs which totaled 18 hours. A high impressed potential was applied to overcome, as much as possible, the interflow of electrolyte and gases from the compartment leakage. A 500-ampere current at a 10-volt potential was used during the first 6 hours of operation. A large volume of effluent TiCl4 was obtained in this initial depletion of the soluble Ti of the anolyte. A satisfactory flow of chlorine was attained at the end of this time and the current was reduced to 250 amperes at 7.5 volts for the following 12 hours of operation. After a 4-hour downtime, the remaining operation was held at 200 amperes and 7 volts for a total of 15,200 ampere-hours, except for two other downtimes.
Chlorine efficiencies taken at intervals were from 65 to 79 percent. These chlorine efficiencies were surprisingly high, considering the adverse condition of intercompartment leakage.
The basket-type, flat, ¾-inch-thick loose material composite diaphragm was constructed of alumina-coated 8- and 20-mesh nickel screens with a minus 80- plus 100-mesh silica fill, and had an exposed area subject to electrolysis of 0.6 ft². The used diaphragm showed no deterioration.
Workability of the cell was considered good; however, the 2-inch-diameter dense graphite anode was permeable to chlorine and leaked profusely; high- temperature silicon cement served as a temporary seal. Some modifications of the cell would be advisable for industrial adaptation.
Conclusions
Two general classes of composite diaphragms were made and tested. Solid materials composite diaphragms (fig. 2) were made of two or more solid component materials, whereas the loose fill materials composite diaphragms (fig, 3) utilized a fill of loose material in addition to the solid materials. One of the most satisfactory diaphragms was diaphragm 14, with a 20-micron-porosity nickel liner with an inner coat of cement. There was no deterioration on the used diaphragm. The highest chlorine efficiencies, practically 100 percent, were obtained with the 12-micron-porosity graphite diaphragm with an inner cement coating (diaphragm 7). This type was, however, subject to the same current density limitation as the porous ceramic diaphragms.
The properties of strength, flexibility, resistance to corrosion, low replacement of Ti in loose materials, and ability to tolerate high current densities were attained in the composite loose fill materials diaphragms. These were improvements over the diaphragms described by Couch and Leone. Sand would be the most economical fill, but would still require replacement, as shown by the 4-percent Ti content that resulted in tests. The 1-percent Ti replacement that occurred in the BN fibers fill was highly favorable and could likely be lowered by the use of granular BN; however, the cost is high. The low 0.2 to 1.0 percent replacement in the alumina granular fill favor this as the preferable fill.
The continuous test series, which was made in the rectangular cell, utilizing a silica-filled flat diaphragm, showed that with modifications the cell could be feasibly adapted for the electrowinning of high-purity titanium from TiCl4.
