Table of Contents
Electrowinning is an electrochemical operation employing an anode and cathode immersed in an electrolytic solution containing sulfuric acid and aqueous copper sulfate. When direct electrical current passes between the cathode and anode through the electrolyte, very pure metallic copper is deposited on the surface of the cathode. The process is conducted commercially in electrolytic cells containing many pairs of anodes and cathodes; a large electrowinning tankhouse, producing 100,000 tonnes cathode copper per year, will typically contain several hundred cells and thousands of anode/cathode pairs. Two principal byproducts, sulfuric acid and oxygen, result from electrowinning. According to the stoichiometry of conventional copper electrowinning, for each kilogram of copper plated, 0.17 Nm³ of oxygen is generated on the anodes, and forms bubbles which rise to the surface of the electrolyte. When these bubbles reach the surface of the electrolyte, they burst, releasing a fine aerosol or mist of electrolyte into the tankhouse environment. Although the total amount of electrolyte (and oxygen) released is quite small, it creates two significant problems: personnel in the tankhouse are exposed to the acid mist; and acid mist is deposited on surfaces inside the tankhouse, such as structural supports, siding, handrails, piping, and equipment, leading to corrosion.
Ventilation
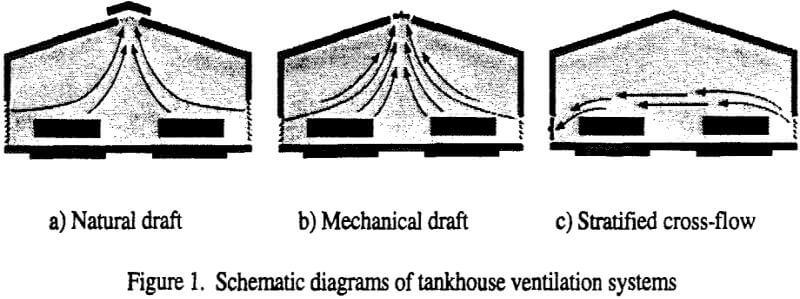
A standard approach to conventional building ventilation is to provide enough fresh air to dilute all contaminants to concentrations below their regulatory limits (Figure 1). If the building interior is well-mixed, the required amount of ventilation fresh air can calculated from the amount of acid generated at the cell tops, the allowable mist concentration and the mixing efficiency. For a medium-sized electrowinning tankhouse, hypothetical values yield the following required air rate:
(200 mg/min-cell) (200 cells)/(0.8 mg/m³) (50%) = 100,000 m³/min (3,500,000 cfm)…………………..(1)
There are four problems with this approach:
- The amount of air required is relatively high, and the installed fan power can approach 20% of the electrowinning power requirement. To overcome the power requirement, natural ventilation has been attempted, but the thermal driving force may be insufficient to move the required amount of air, especially in warmer climates. The power requirement can also be overcome by operating an open building (roof but no siding). This works to dilute the acid mist when there is a wind in moderate climates. In arid and windy climates, copper quality may suffer due to dust entering and settling in the cells. In colder climates, especially those with large diurnal temperature variation, it becomes difficult to maintain the correct electrolyte temperature, and in extreme cold climates both difficult and expensive to meet space heating requirements.
- The mixing efficiency is either unknown or at best difficult to establish by either physical or mathematical models or extrapolation from other tankhouses, especially when external wind effects are superimposed on the tankhouse.
- The acid mist generation rate is not well-known, but depends on the electrolyte strength and surface tension, operating temperature, current density, and age-related anode surface conditions.
- Some of the air exhausted from the tankhouse will be carried back and re-entrained into the building air inlets. Modeling results indicated that at certain moderate wind speeds the re-entrainment may be higher than 40%. Of course, this greatly increases the air flow required to obtain the necessary fresh dilution air.
Cell Cap Ventilation System
Reviewing the above options for controlling electrowinning tankhouse acid mist leads to a conclusion that none of the options is well-suited for meeting today’s regulations. In fact, several of the options are most often used in combination, and even then, operators are sometimes required to wear respirators. If the future brings more stringent regulations, the above measures may not be adequate. Recognizing that all the existing technologies suffer from significant disadvantages, Bechtel sought to develop a new approach which would meet the following requirements:
- Reduce the acid mist level in the tankhouse to levels previously unattainable, in order to meet expected future work safety regulations
- Capture generated acid mist in order to control emissions to the environment outside of the tankhouse
- Not increase the operating cost of the tankhouse
- Compatibility with modern permanent cathode and lead anode handling operations
- Applicable to retrofitting plants currently employing other acid mist control techniques
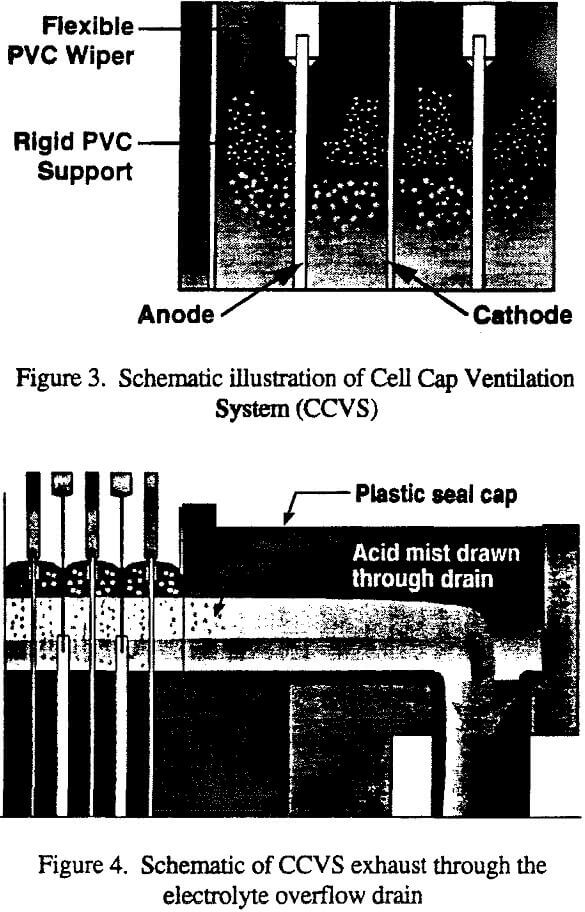
One of the main principles of limiting work place concentrations of pollutants is to control any emission as close to the source as possible. From this basis, further development of a cell cover design tried. At that time, non-conductive wipers were attached to each side of the anodes, above the electrolyte level in the electrowinning cell, and cathodes slipped through the gap between two adjacent wipers. The wipers did not extend to the sides of the cells, and because the cells were not completely covered, acid mist was able to drift under the wipers into the tankhouse work areas. Nonetheless, the non-conductive wipers were the starting point of the new design because they captured acid mist closest to its source. The new Cell Cap Ventilation System (CCVS) includes the following features:
- A system of PVC wiper blades, illustrated in Figure 3, attached to the anodes to form a cover over the electrolyte. The wipers allow permanent stainless steel cathodes to be harvested and re-inserted without removing or reducing the effectiveness of the cover.
- At each end of the wipers are flexible PVC tabs which reach to the sides of the cell. Because the tabs are flexible, they seal against irregularities of the polymer concrete, paraliner cells, or buffer sheets.
- The inlet and overflow ends of the cells are fitted with PVC pieces that cover from the wipers of the last anode- to the cell end. The cup over the drain is also covered.
- The oxygen and vapor released between the electrolyte surface and the cover described in (1) through (3) above must be vented to preclude an increase in pressure under the cell cap, which could allow the escape of mist to the tankhouse. It was determined that the electrolyte drain system could be designed to convey simultaneously the electrolyte in the bottom portion of the pipe, and the venting gases in the upper portion.
- The sulfuric acid mist is disengaged from the exhaust gases before they are vented to atmosphere. Most of the aerosol is expected to settle in the drain piping.
- Conventional building ventilation is applied to the tankhouse, at a fraction of the air rate for stratified systems, which in the coldest climates results in much reduced heating requirements.

